epidural kit price
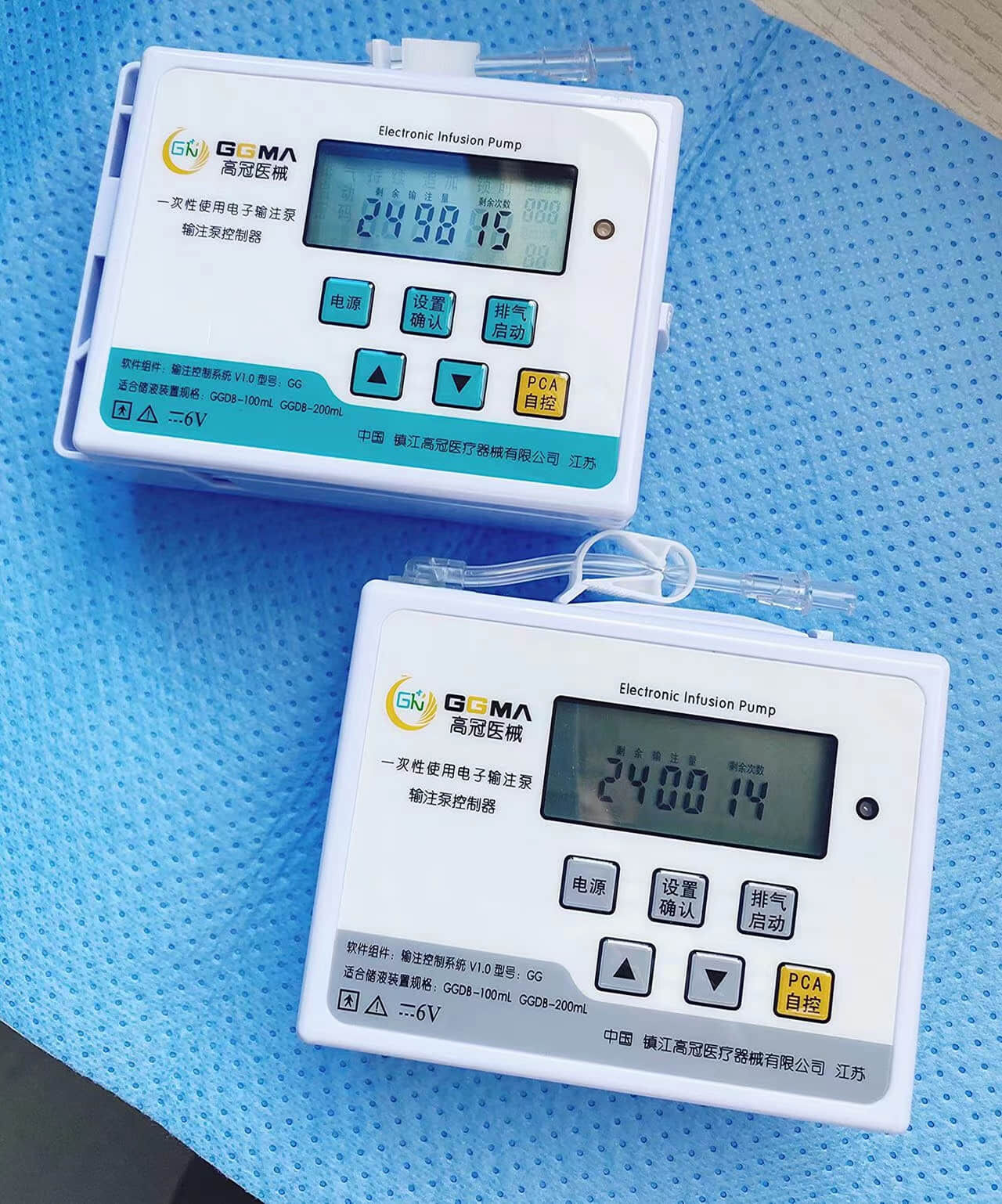
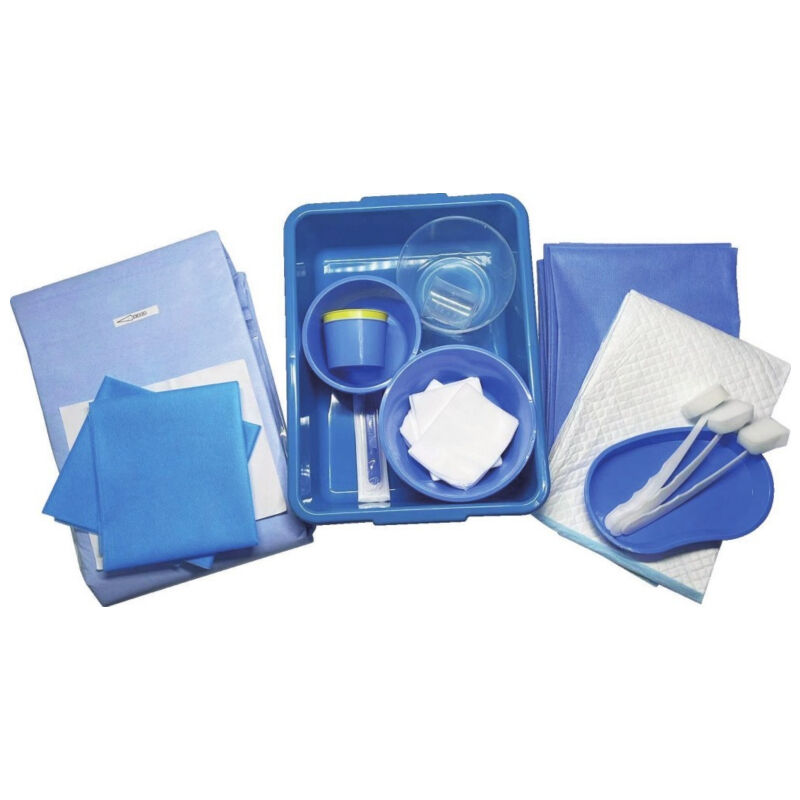
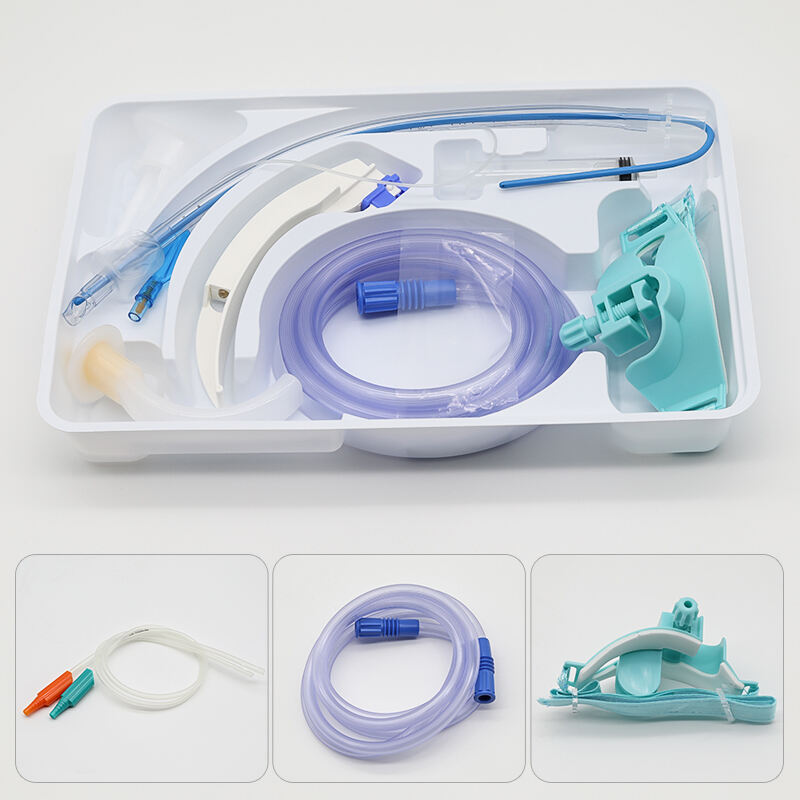
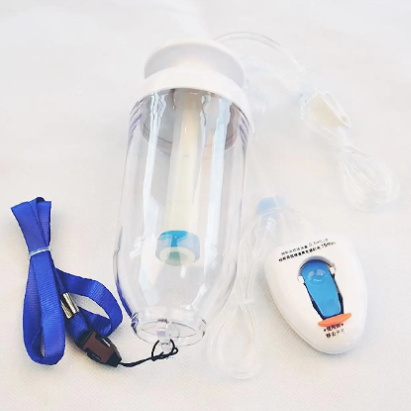
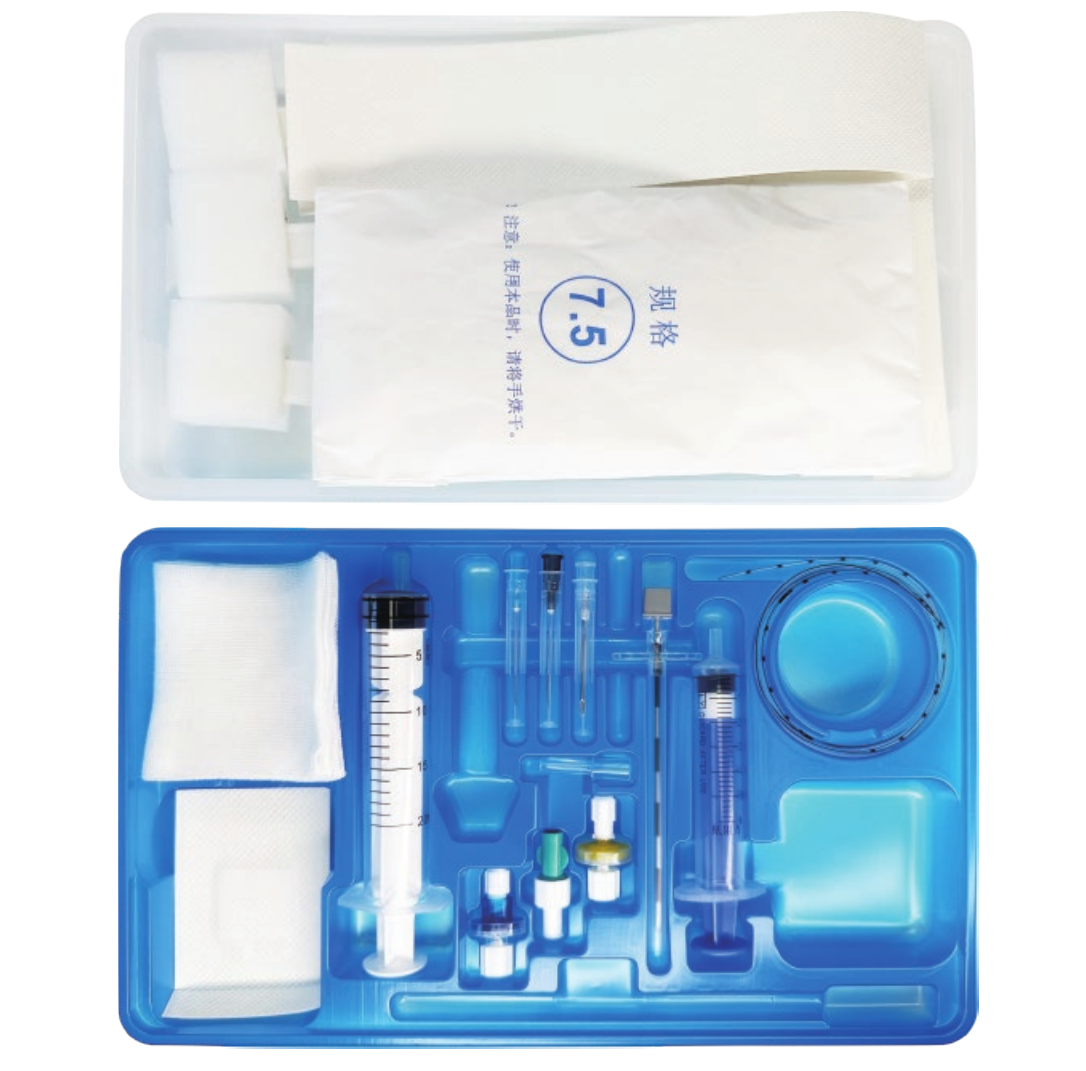
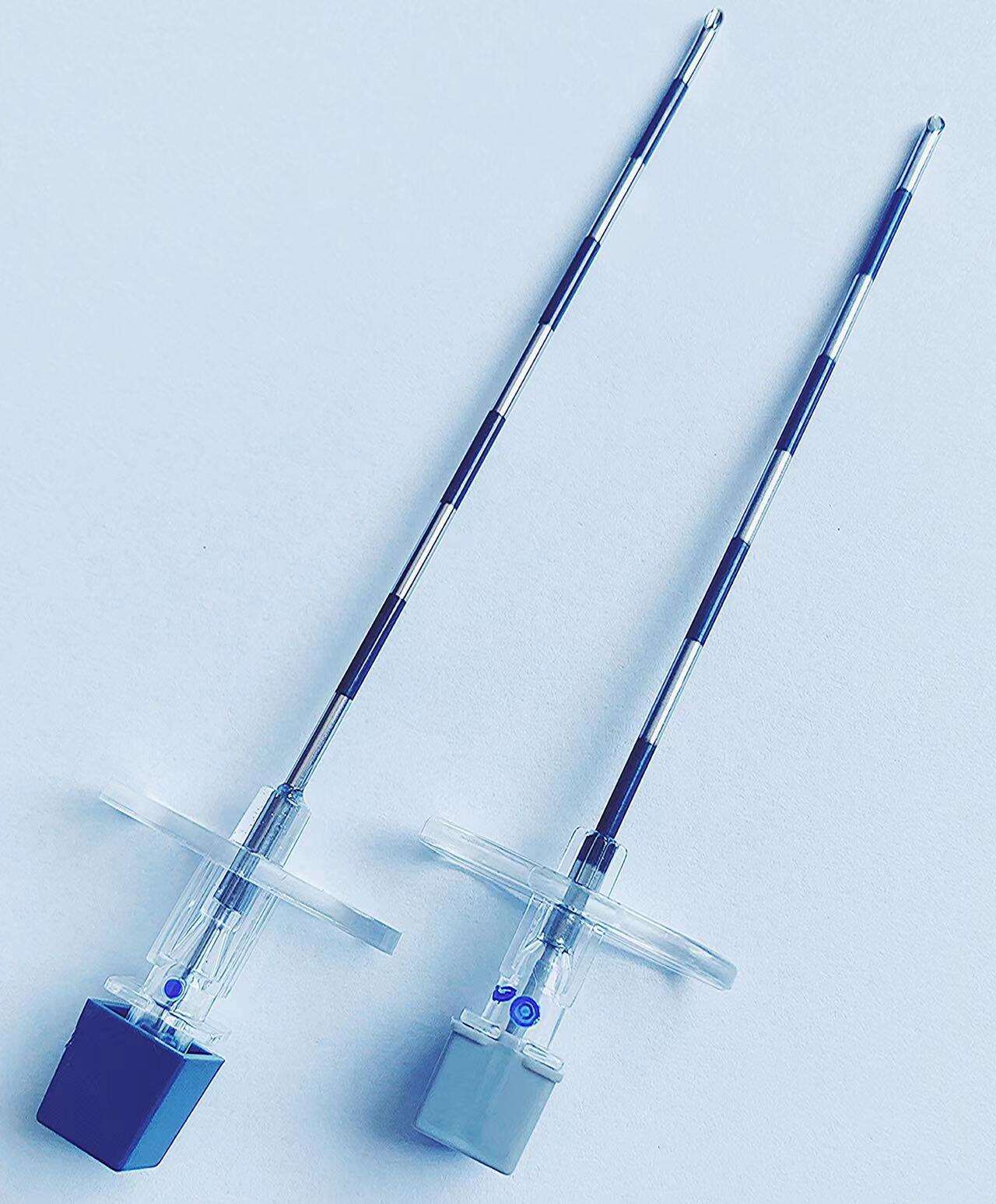
Understanding the epidural kit price involves examining a comprehensive medical package designed for administering epidural anesthesia and analgesia procedures. These specialized kits contain essential components that healthcare professionals require to perform safe and effective epidural injections in clinical settings. The epidural kit price reflects the sophisticated manufacturing standards, quality control measures, and regulatory compliance necessary for medical-grade equipment used in critical pain management procedures. Modern epidural kits typically include sterile needles, catheters, syringes, local anesthetic solutions, and positioning aids that ensure precise medication delivery to the epidural space surrounding the spinal cord. The technological features incorporated into contemporary epidural kits demonstrate significant advancement in medical device engineering. Many kits feature Tuohy needles with enhanced tip designs that provide tactile feedback when penetrating tissue layers, reducing the risk of accidental dural puncture. Advanced catheter materials offer improved flexibility and radiopacity, allowing for better visualization under fluoroscopic guidance. Some premium epidural kits include integrated pressure monitoring systems that help clinicians identify correct needle placement through real-time feedback mechanisms. The epidural kit price often correlates with these technological enhancements, as more sophisticated components require additional research, development, and manufacturing precision. Applications for epidural kits span multiple medical specialties, including obstetrics for labor pain management, orthopedic surgery for postoperative pain control, and chronic pain management in specialized clinics. In obstetric settings, epidural kits enable expectant mothers to experience reduced labor pain while maintaining consciousness and participation in the birthing process. Surgical applications utilize epidural anesthesia to provide regional anesthesia for procedures involving the lower extremities, abdomen, and pelvis. The versatility of epidural kit applications contributes to market demand and influences pricing structures across different healthcare sectors. Quality assurance protocols significantly impact the epidural kit price, as manufacturers must adhere to stringent FDA regulations and international medical device standards. Each component undergoes rigorous testing for sterility, biocompatibility, and functional performance before packaging.