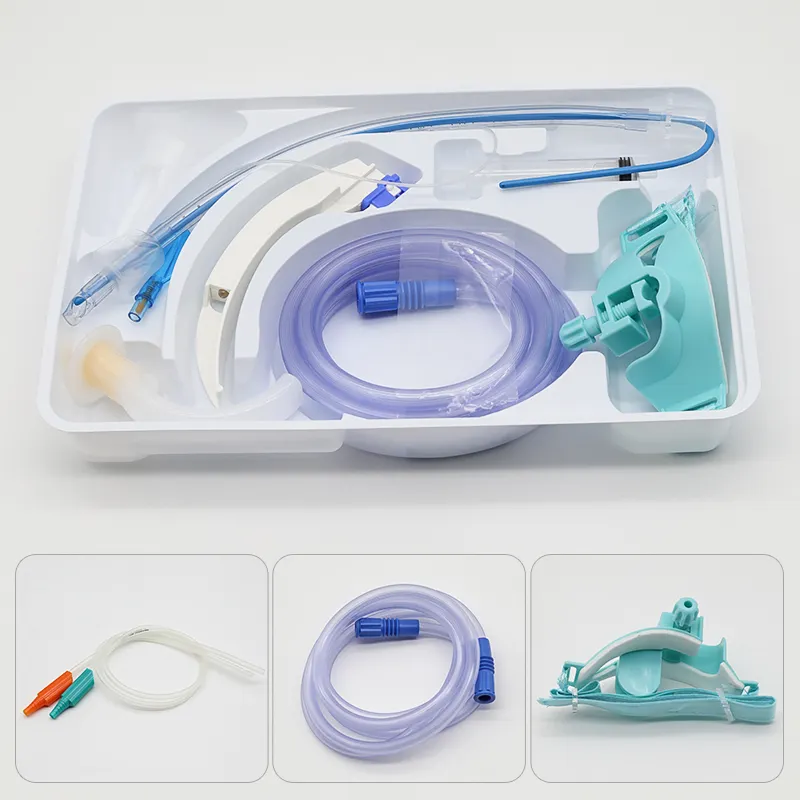
Superior Manufacturing Quality Delivers Consistent Clinical Performance
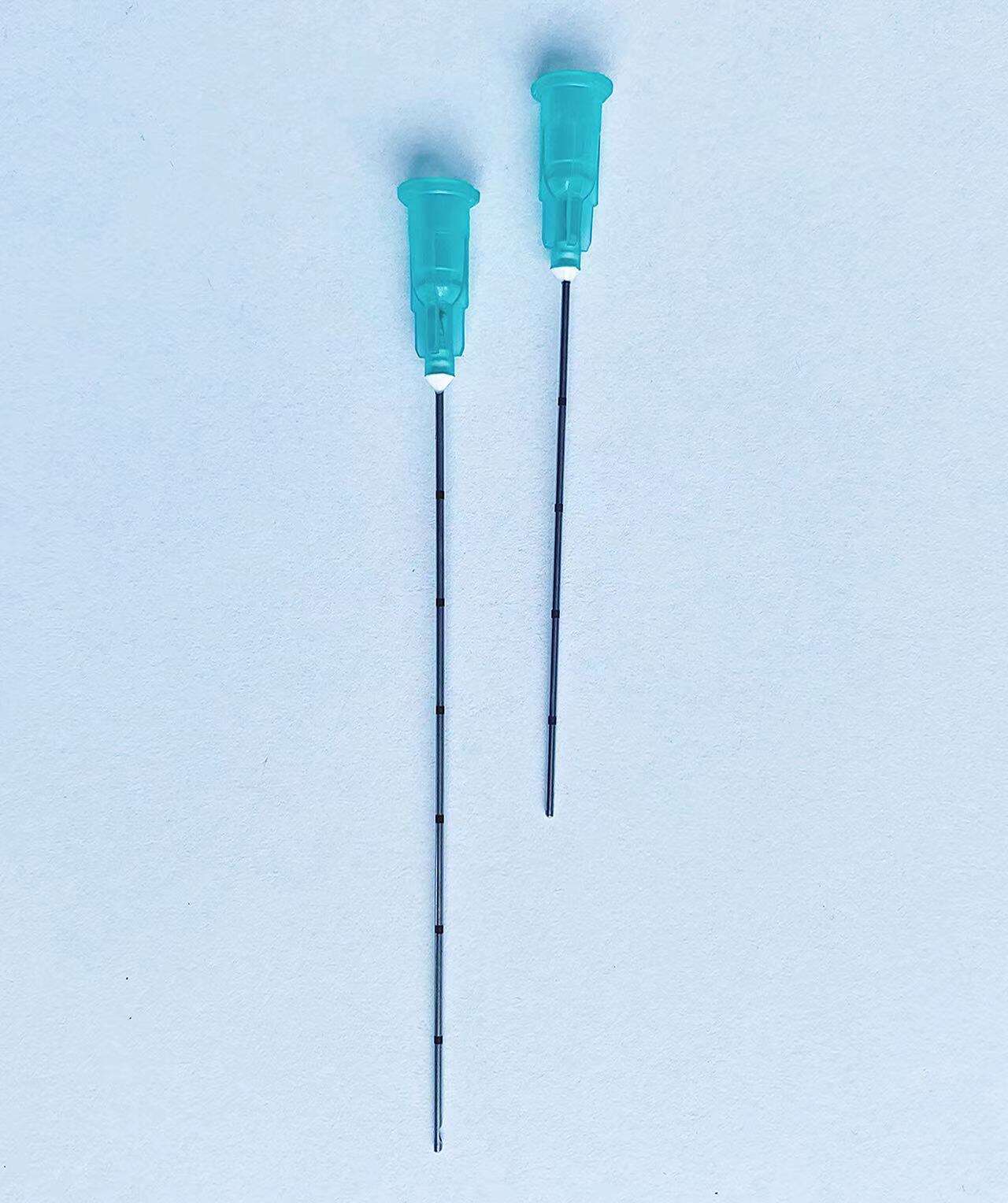
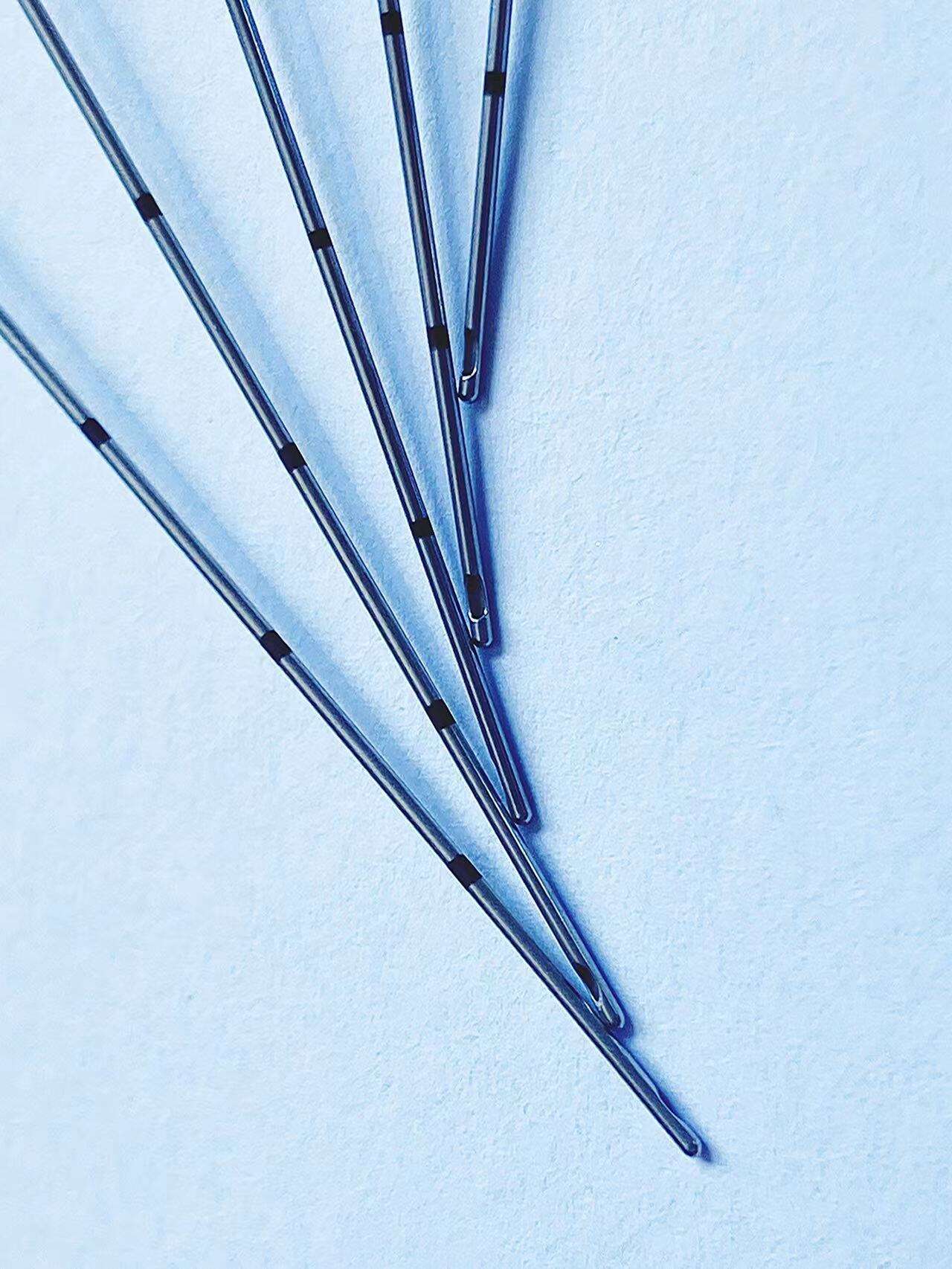
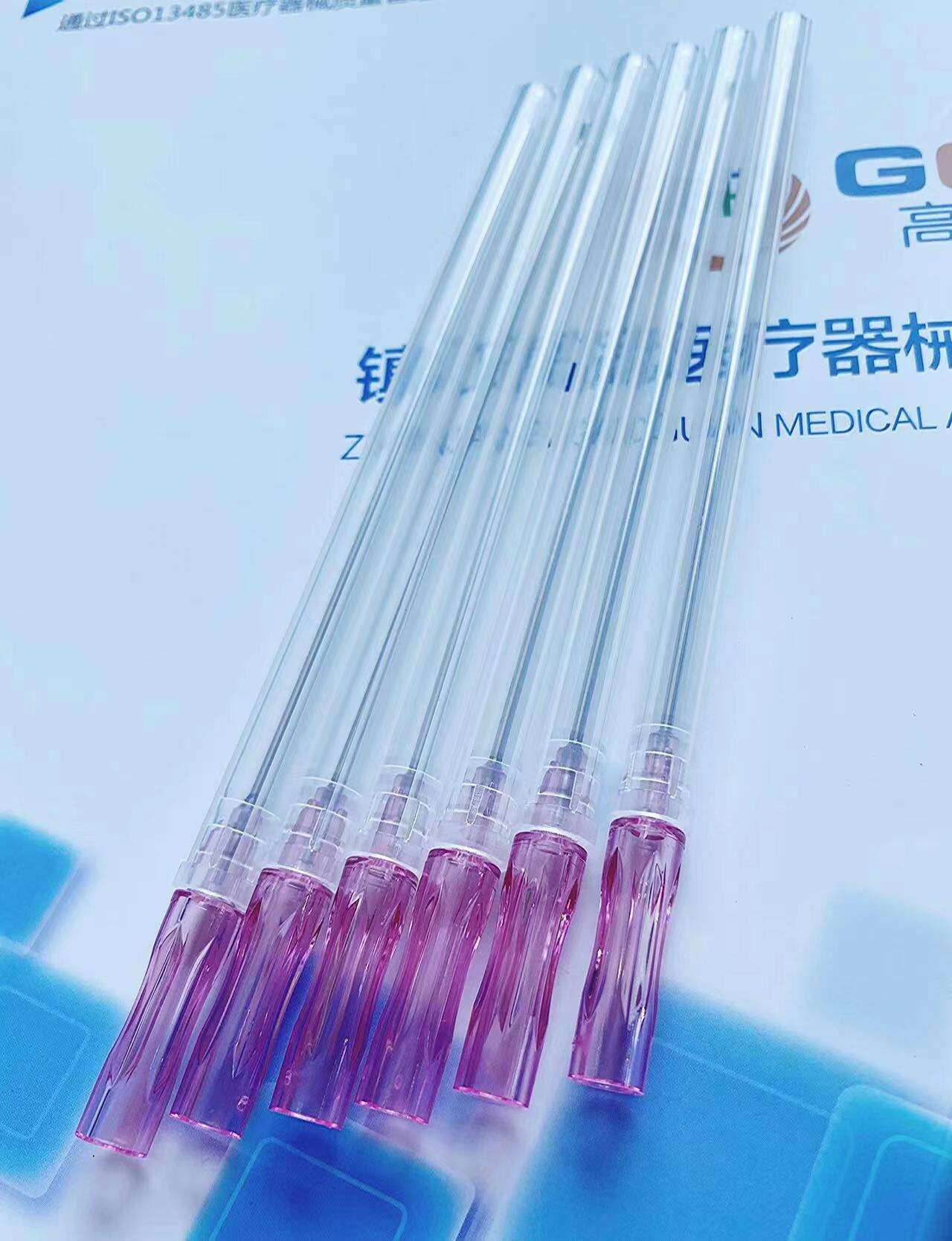
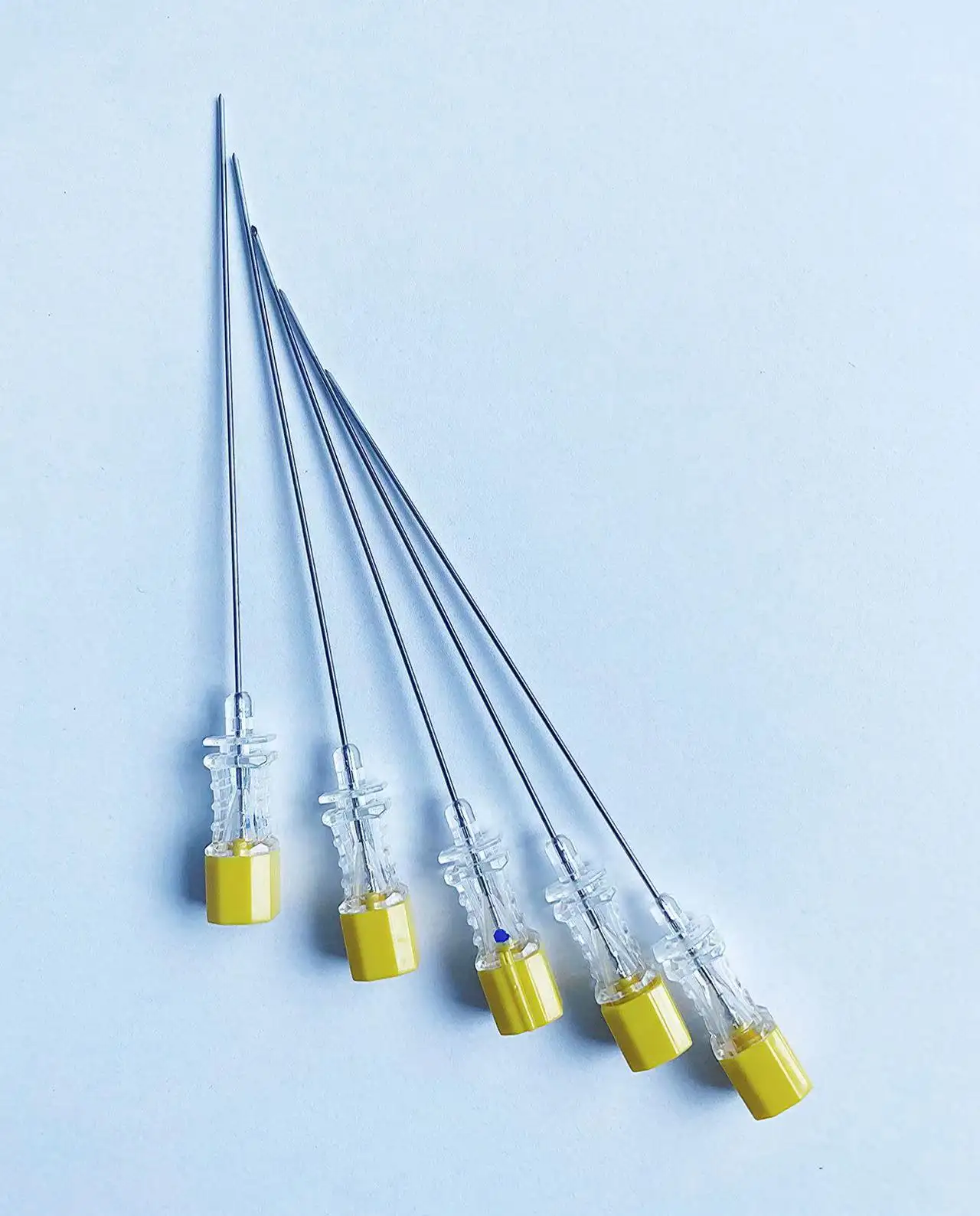
The pencil point spinal needle with introducer exemplifies manufacturing excellence through advanced production techniques, premium materials, and rigorous quality control measures that ensure consistent clinical performance and patient safety. Each needle undergoes a sophisticated manufacturing process that begins with high-grade stainless steel selection, chosen specifically for its biocompatibility, strength, and corrosion resistance properties. The steel alloy used in the pencil point spinal needle with introducer meets stringent medical device standards and undergoes comprehensive testing to verify chemical composition, mechanical properties, and surface characteristics. The precision machining process employed to create the distinctive pencil point geometry requires state-of-the-art equipment capable of achieving tolerances measured in micrometers. Computer-controlled manufacturing systems ensure that every pencil point spinal needle with introducer maintains identical tip angles, surface smoothness, and dimensional accuracy. The critical pencil point tip undergoes specialized polishing procedures that eliminate microscopic imperfections, creating an ultra-smooth surface that glides through tissue with minimal resistance. Quality control measures for the pencil point spinal needle with introducer include automated inspection systems that verify tip geometry using high-resolution optical measurement techniques. Each needle passes through multiple quality checkpoints, including dimensional verification, surface finish analysis, and functional testing. The introducer component receives equal attention during manufacturing, with precision boring operations ensuring proper internal dimensions and smooth needle passage. Sterilization processes for the pencil point spinal needle with introducer utilize validated gamma radiation or ethylene oxide methods that eliminate all microorganisms while preserving needle integrity and performance characteristics. Packaging systems incorporate barrier materials that maintain sterility throughout storage and transportation while providing easy access during clinical use. Traceability systems track each pencil point spinal needle with introducer from raw material receipt through final packaging, enabling comprehensive quality documentation and rapid response to any quality concerns. This commitment to manufacturing excellence translates directly into clinical benefits, as healthcare providers can rely on consistent performance, predictable handling characteristics, and optimal patient outcomes with every procedure involving the pencil point spinal needle with introducer.