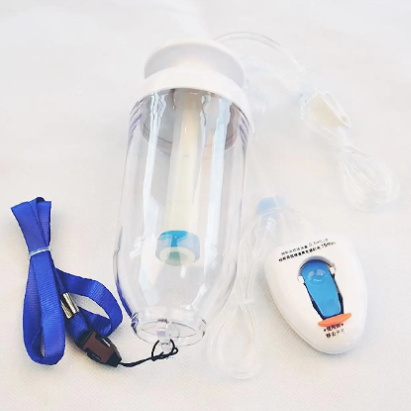
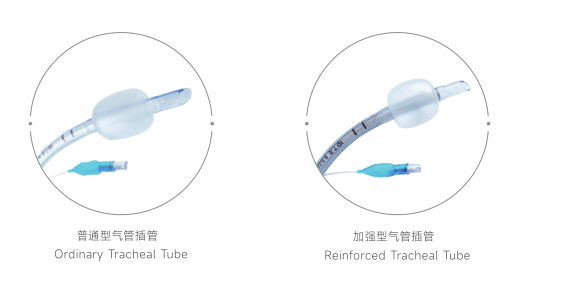
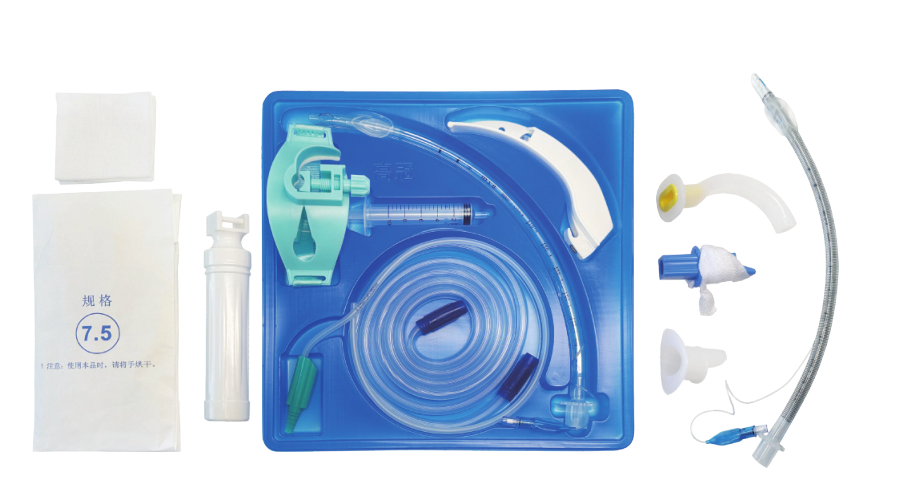
Universal Compatibility Framework
The universal compatibility framework embedded within endotracheal tube kits revolutionizes medical equipment integration by ensuring seamless connectivity across diverse hospital systems and equipment manufacturers. This innovative approach eliminates the frustrating compatibility issues that historically plagued medical facilities using equipment from multiple vendors. Healthcare professionals benefit from standardized connection protocols that allow endotracheal tube kits to interface effectively with various ventilation systems, monitoring equipment, and life support devices regardless of manufacturer specifications. The engineering excellence behind this universal compatibility extends to dimensional standardization, ensuring that endotracheal tube kits fit properly within existing medical infrastructure without requiring costly modifications or specialized adapters. Medical facilities appreciate the flexibility that universal compatibility provides, allowing them to optimize equipment procurement strategies based on clinical needs rather than technical limitations. The compatibility framework includes comprehensive testing protocols that verify performance across different environmental conditions and equipment configurations commonly encountered in modern healthcare settings. This thorough validation process ensures that endotracheal tube kits maintain optimal functionality whether used in emergency departments, operating theaters, intensive care units, or mobile medical facilities. Healthcare administrators value the reduced training requirements associated with universal compatibility, as staff members can apply their existing knowledge across different equipment platforms without extensive retraining programs. The forward-thinking design philosophy incorporates anticipated technological developments, ensuring that current endotracheal tube kit investments remain viable as medical technology continues to evolve and advance.