disposable intervention surgical bag factory
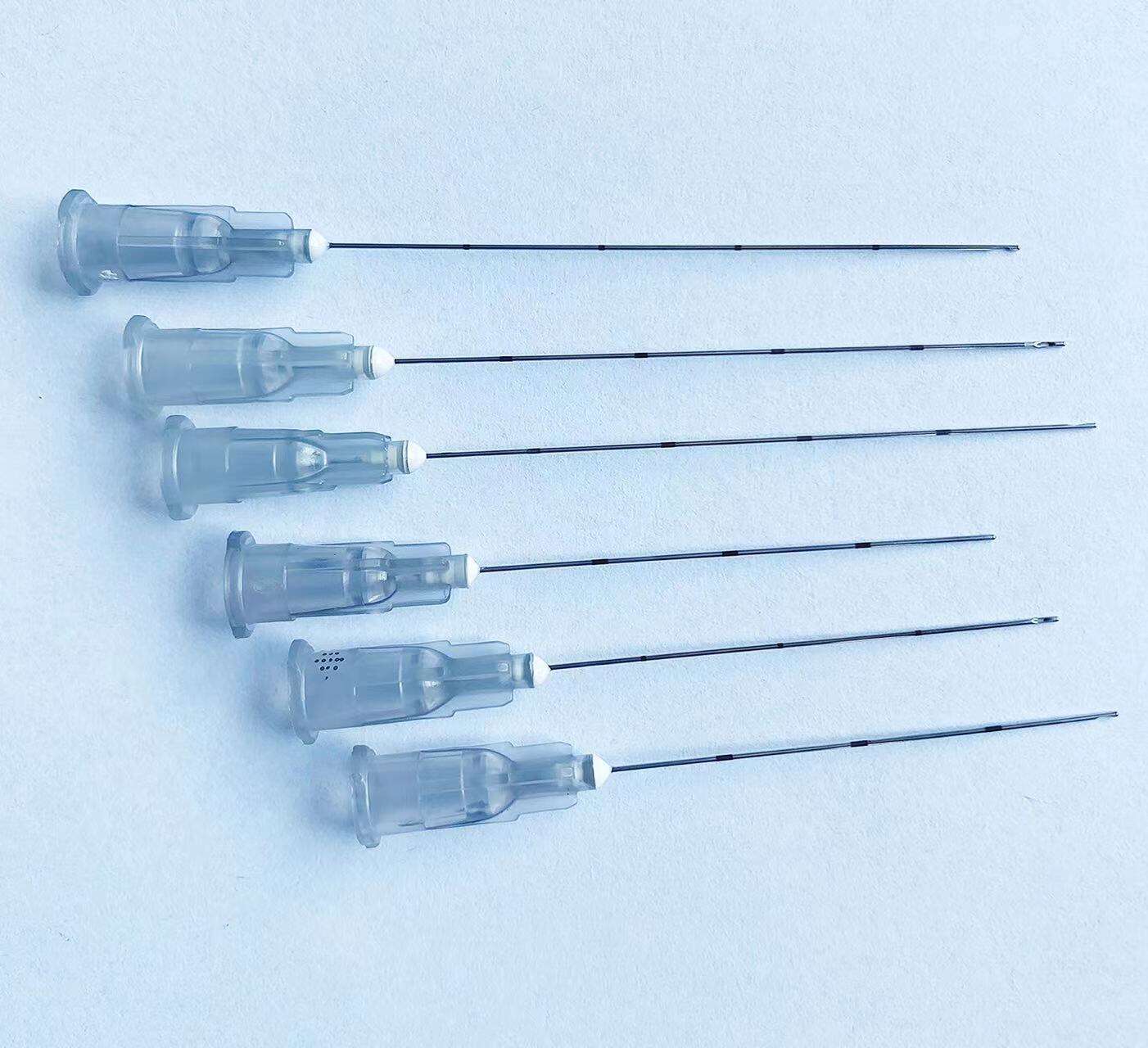
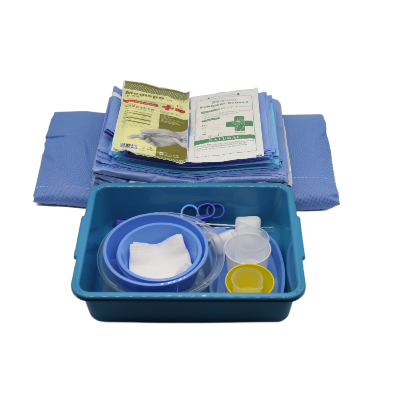
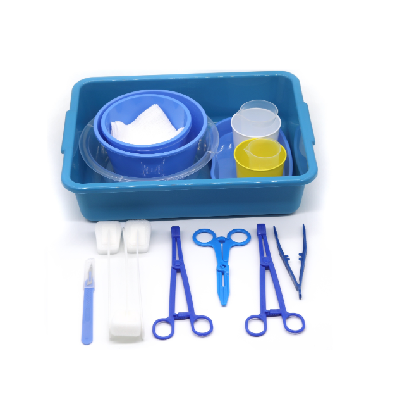
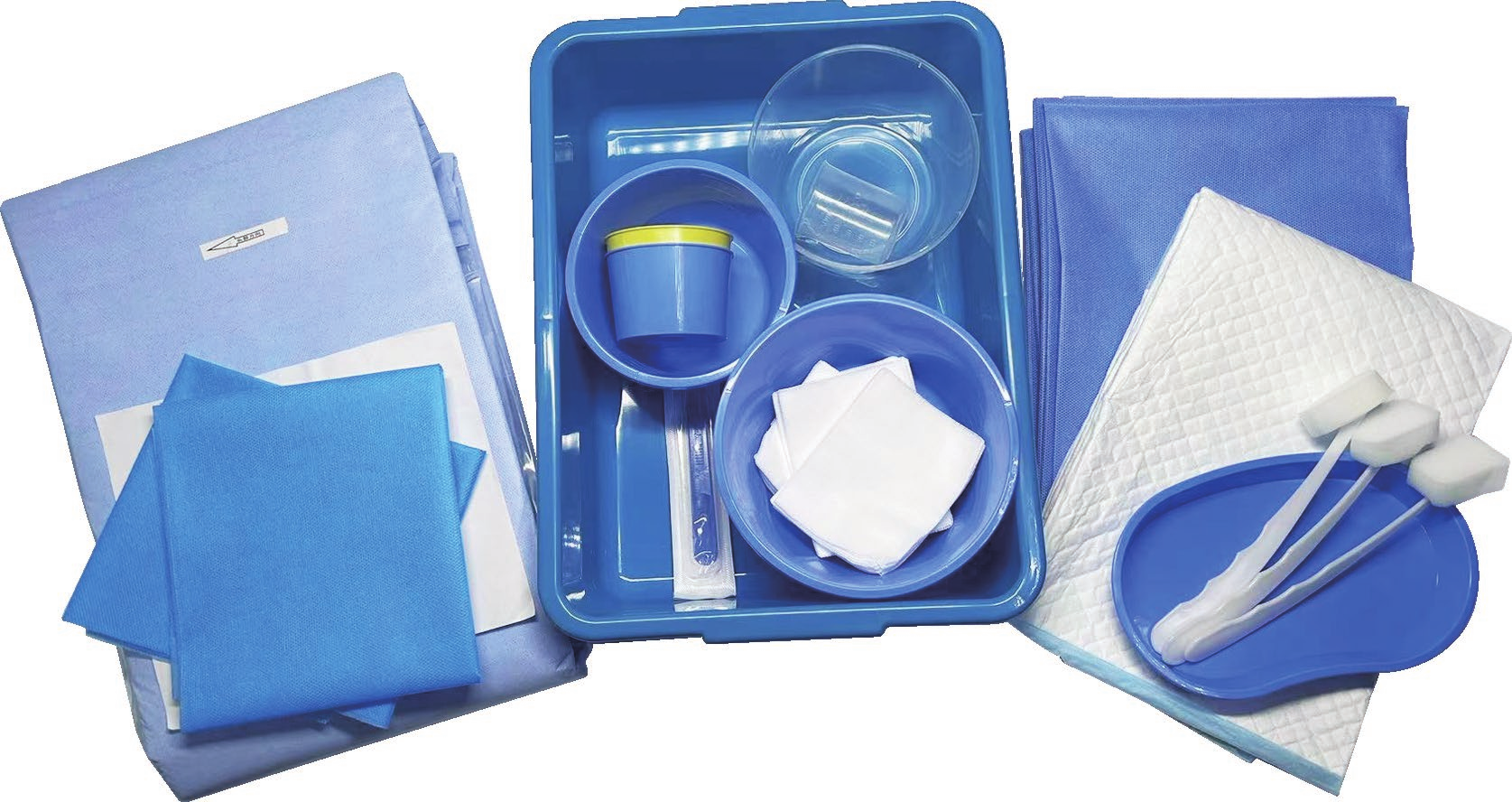
A disposable intervention surgical bag factory represents a specialized manufacturing facility dedicated to producing sterile, single-use surgical draping systems designed specifically for interventional procedures. These state-of-the-art production facilities combine advanced manufacturing technologies with stringent quality control measures to create comprehensive surgical bag solutions that meet the demanding requirements of modern healthcare environments. The disposable intervention surgical bag factory utilizes cutting-edge automation systems, including precision cutting machinery, ultrasonic welding equipment, and sterile packaging lines that ensure consistent product quality while maintaining the highest standards of contamination prevention. The primary function of a disposable intervention surgical bag factory centers around manufacturing complete surgical draping kits that provide optimal sterile barriers during complex interventional procedures such as cardiac catheterization, angioplasty, and minimally invasive surgeries. These facilities incorporate sophisticated clean room environments that adhere to ISO 13485 medical device manufacturing standards, featuring HEPA filtration systems, positive air pressure controls, and temperature-regulated production zones. The technological features of a disposable intervention surgical bag factory include automated material handling systems that process medical-grade nonwoven fabrics, plastic films, and adhesive components while maintaining sterile conditions throughout the manufacturing process. Advanced quality assurance protocols involve real-time monitoring systems that track production parameters, conduct bacterial testing, and perform integrity assessments on every batch produced. The applications of products manufactured by a disposable intervention surgical bag factory extend across multiple medical specialties including cardiovascular surgery, orthopedic procedures, neurosurgery, and general surgical interventions where maintaining sterile fields is critical for patient safety and successful outcomes.