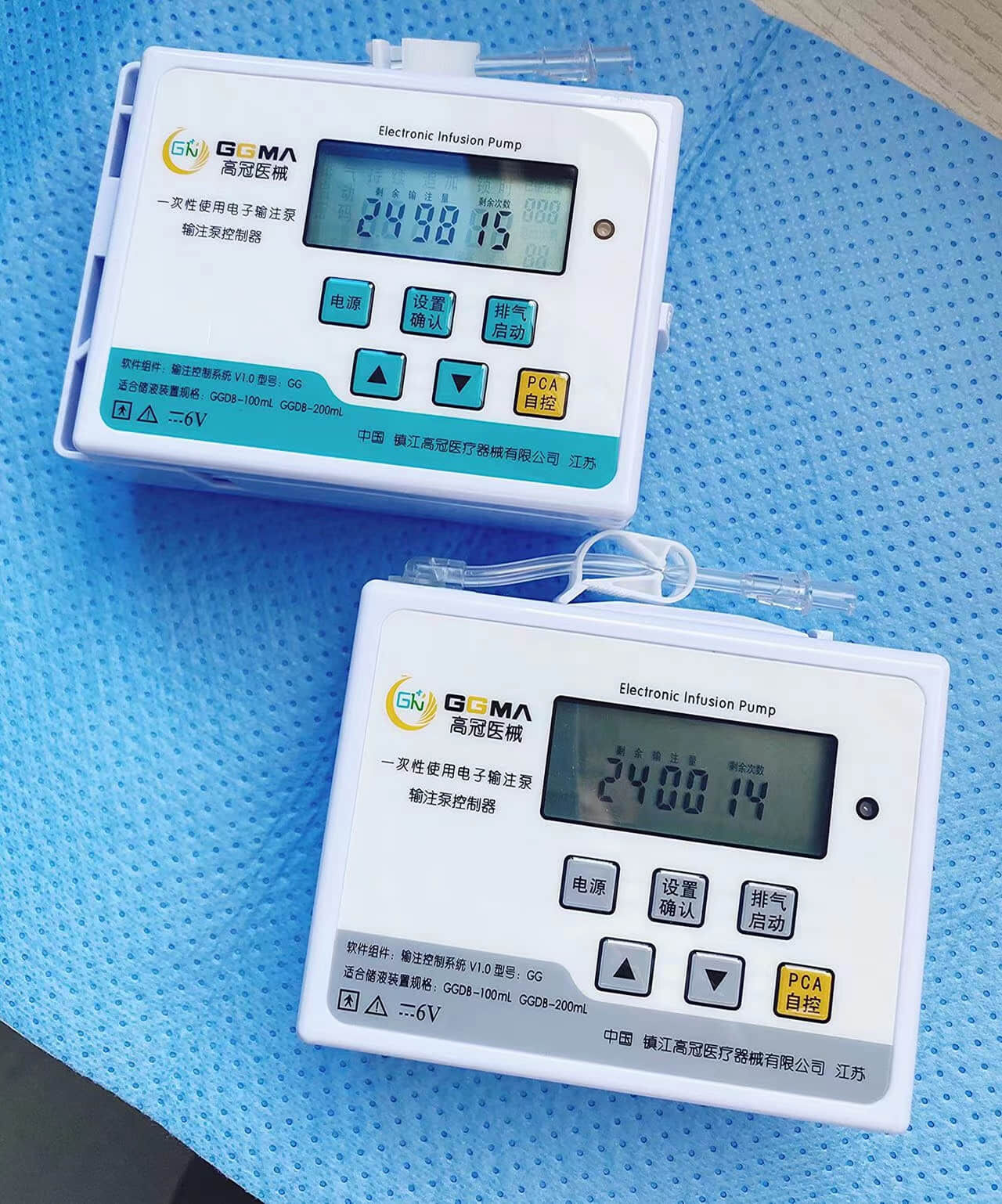
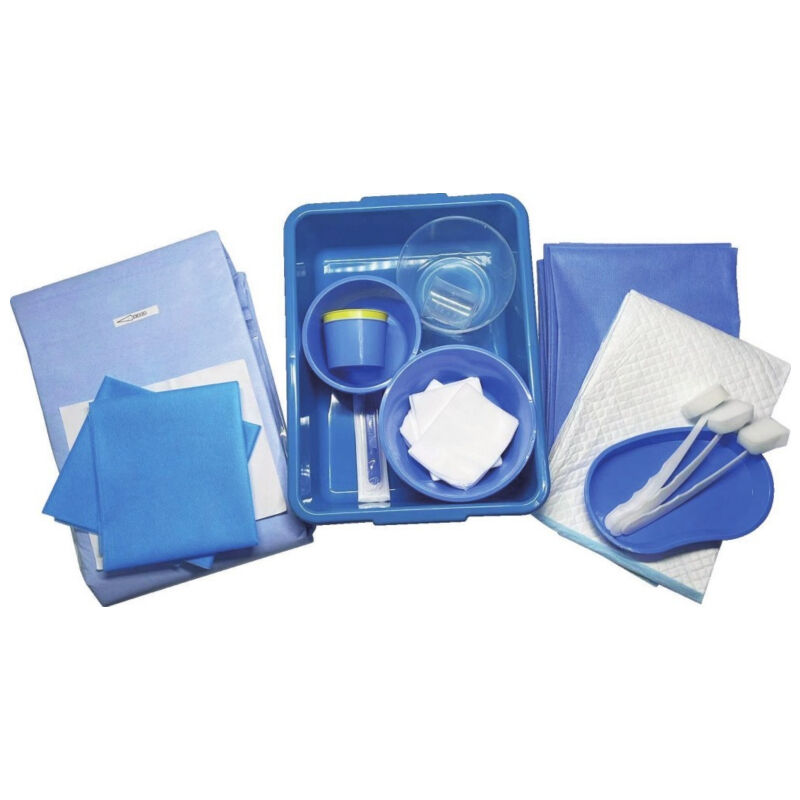
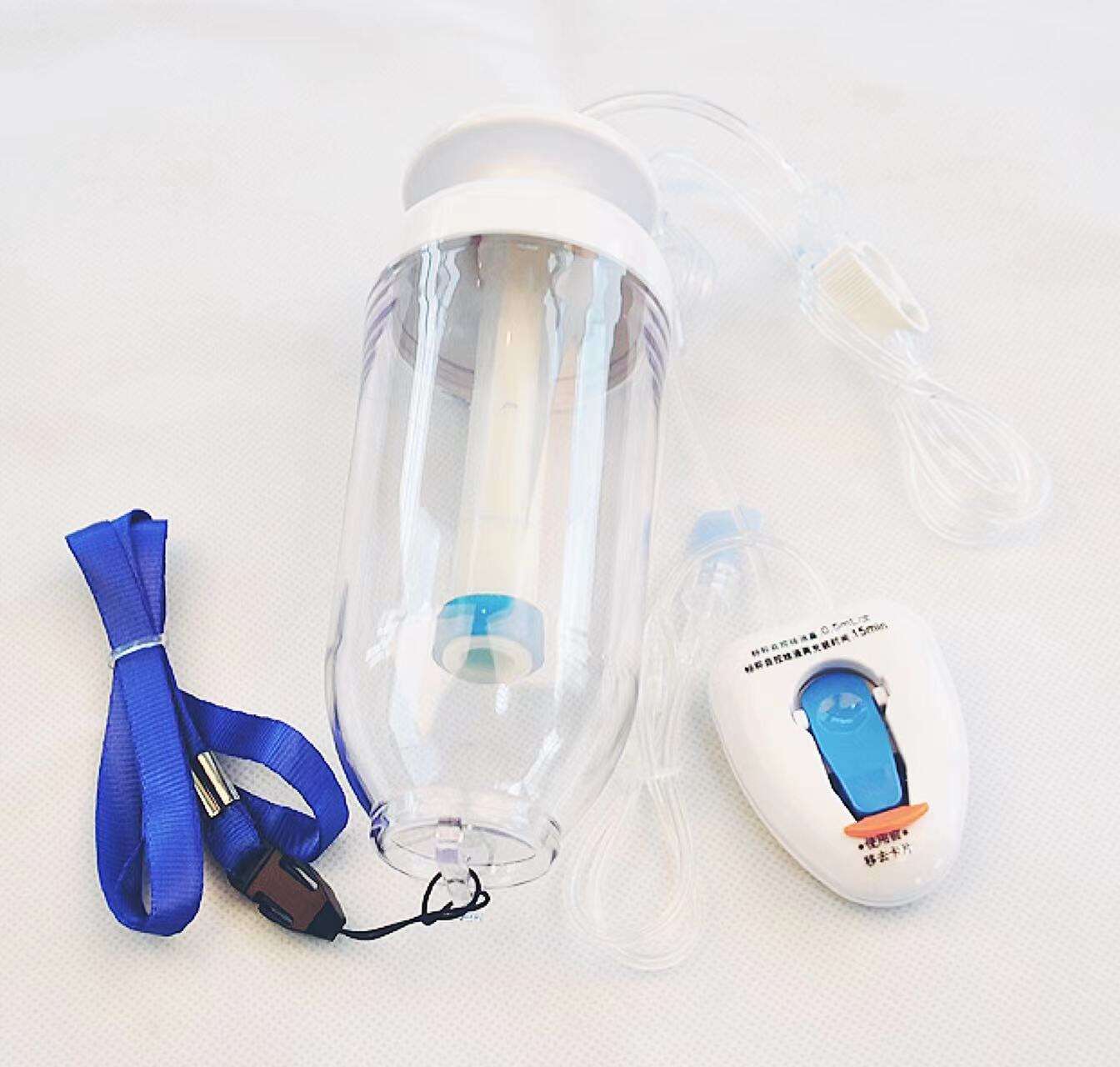
intervention surgical bag supplier
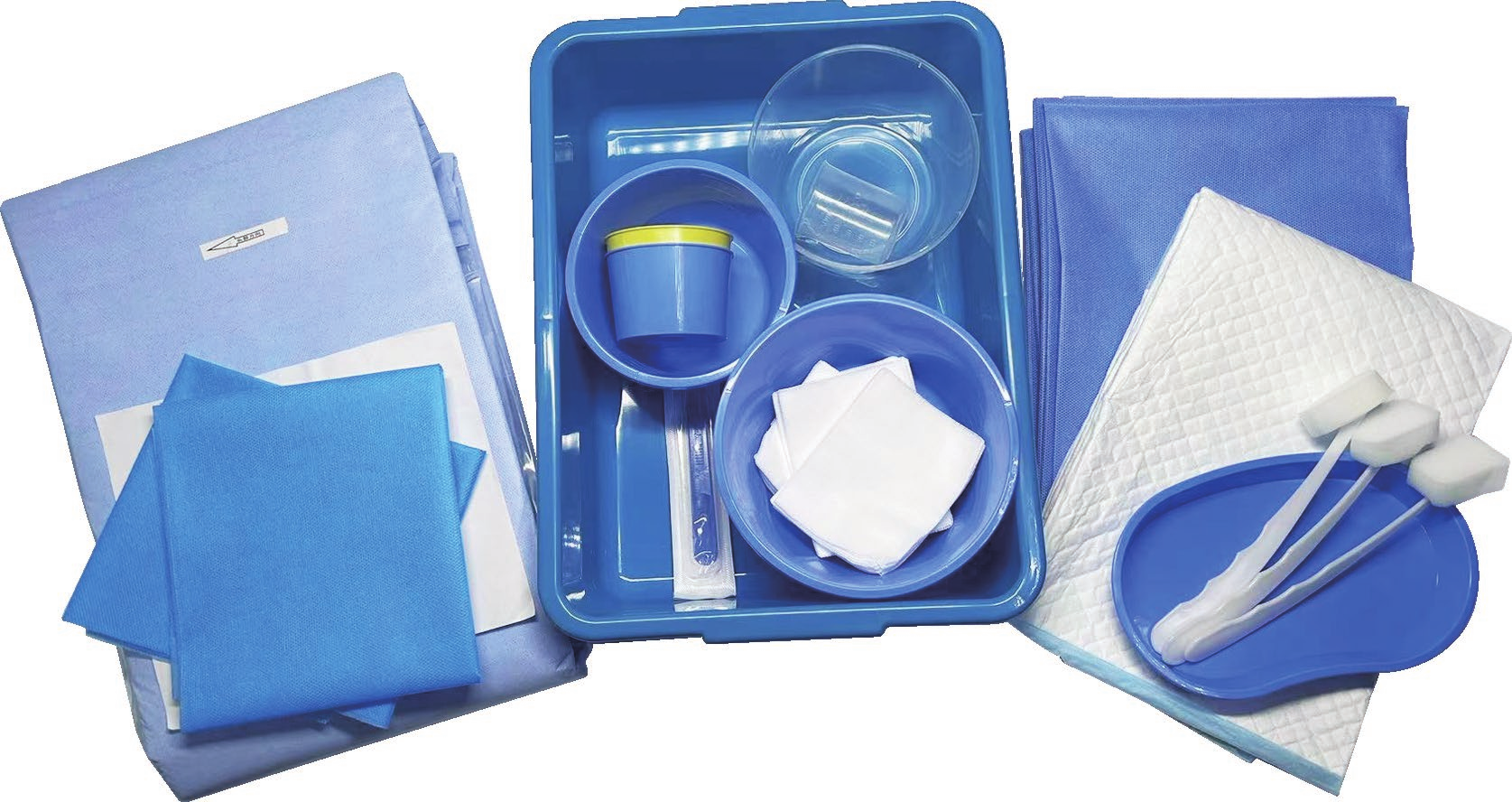
An intervention surgical bag supplier represents a critical component in modern healthcare infrastructure, providing specialized medical packaging solutions designed specifically for interventional procedures. These suppliers deliver comprehensive packaging systems that ensure sterility, organization, and efficiency during complex medical interventions. The intervention surgical bag supplier focuses on creating customized surgical kits that contain all necessary instruments, devices, and materials required for specific interventional procedures, ranging from cardiac catheterizations to minimally invasive surgeries. The primary function of an intervention surgical bag supplier involves manufacturing and distributing sterile, pre-packaged surgical sets that streamline procedural workflows while maintaining the highest standards of safety and contamination control. These suppliers utilize advanced sterilization technologies, including gamma radiation, ethylene oxide, and steam sterilization methods, ensuring complete elimination of pathogens and microorganisms. The technological features incorporated by leading intervention surgical bag suppliers include state-of-the-art packaging materials that provide superior barrier protection, moisture resistance, and durability during storage and transportation. Advanced tracking systems enable real-time inventory management, expiration date monitoring, and supply chain optimization. The applications for intervention surgical bag supplier products span across multiple medical specialties, including cardiovascular surgery, neurosurgery, orthopedics, gastroenterology, and emergency medicine. Hospitals, ambulatory surgical centers, and specialty clinics rely heavily on these suppliers to maintain consistent inventory levels of procedure-specific surgical kits. The intervention surgical bag supplier industry has evolved to meet increasing demands for cost-effective, standardized surgical solutions that reduce preparation time and minimize the risk of procedural delays. These suppliers work closely with healthcare professionals to develop customized packaging solutions that address specific institutional needs and procedural requirements, ensuring optimal patient outcomes and operational efficiency.