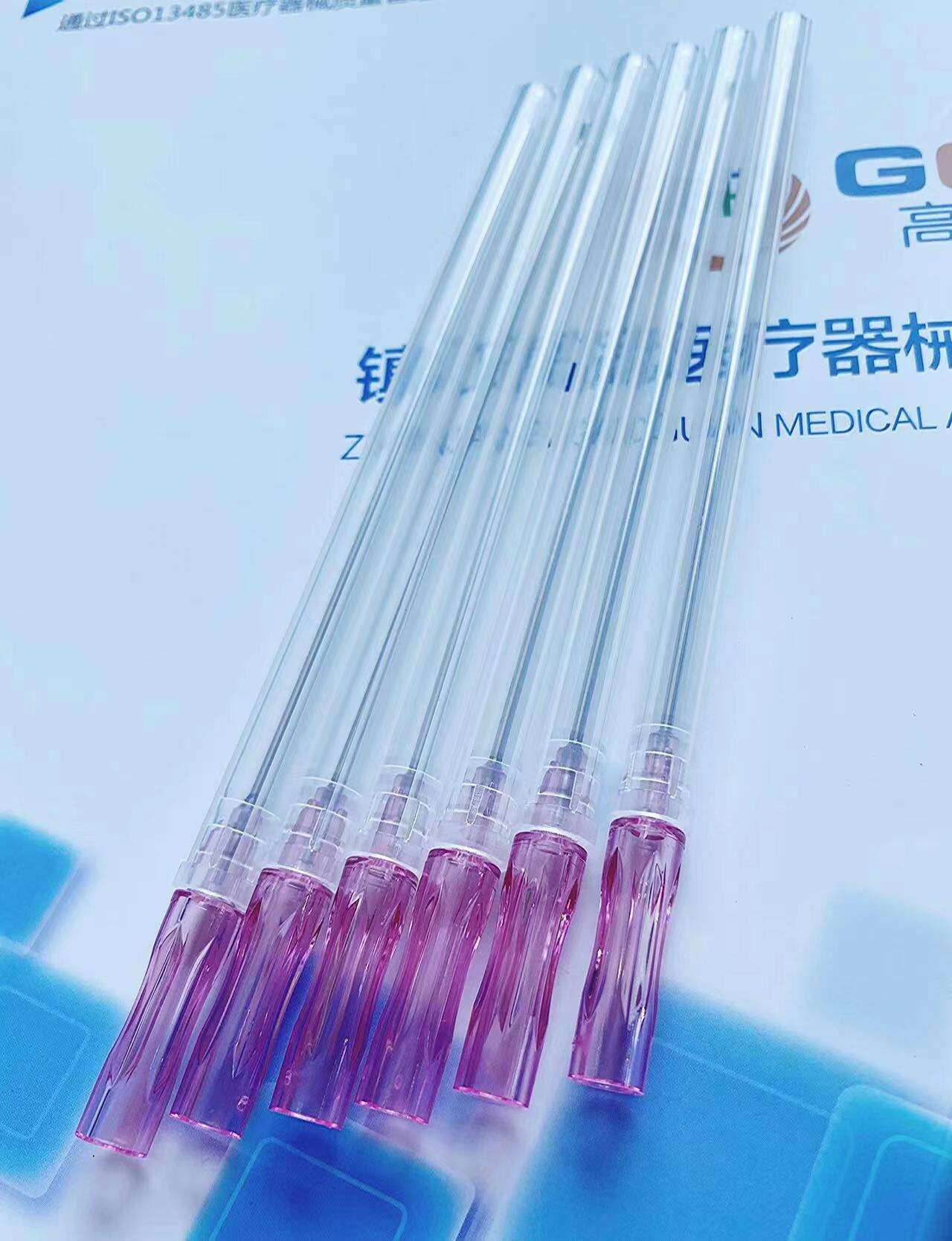
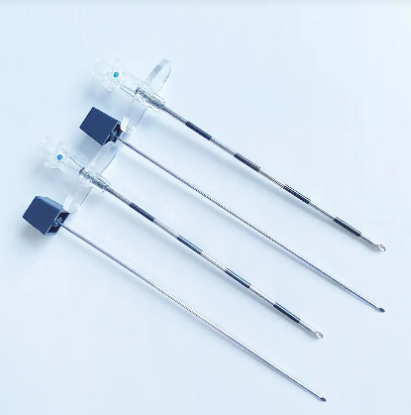
quincke tip needle factory
The quincke tip needle factory represents a specialized manufacturing facility dedicated to producing high-precision spinal needles with distinctive quincke-point designs. These sophisticated medical devices feature a unique cutting-edge configuration that revolutionizes spinal puncture procedures across healthcare institutions worldwide. The quincke tip needle factory employs advanced metallurgical processes, precision engineering techniques, and stringent quality control systems to manufacture needles that meet the exacting standards of modern medical practice. The facility integrates state-of-the-art machinery with expert craftsmanship to produce needles featuring sharp, beveled cutting edges that facilitate smooth tissue penetration during lumbar punctures, epidural procedures, and cerebrospinal fluid collection. The manufacturing process within the quincke tip needle factory involves multiple stages including raw material selection, precision grinding, heat treatment, surface finishing, and comprehensive testing protocols. Each needle undergoes rigorous quality assessments to ensure optimal sharpness, durability, and biocompatibility. The factory utilizes computer-controlled grinding systems that create the characteristic quincke point geometry, which consists of a sharp, diamond-shaped cutting tip that minimizes tissue trauma while maximizing penetration efficiency. Advanced coating technologies are employed to enhance needle surface properties, reducing friction and improving patient comfort during procedures. The quincke tip needle factory serves diverse medical applications including anesthesiology, neurology, pain management, and diagnostic procedures. These needles are essential tools for spinal anesthesia, epidural injections, myelography, and cerebrospinal fluid sampling. The factory maintains strict adherence to international medical device regulations, ensuring that every needle meets FDA, CE marking, and ISO certification requirements. Quality management systems within the facility encompass comprehensive documentation, traceability protocols, and continuous improvement initiatives that guarantee consistent product performance and safety standards.