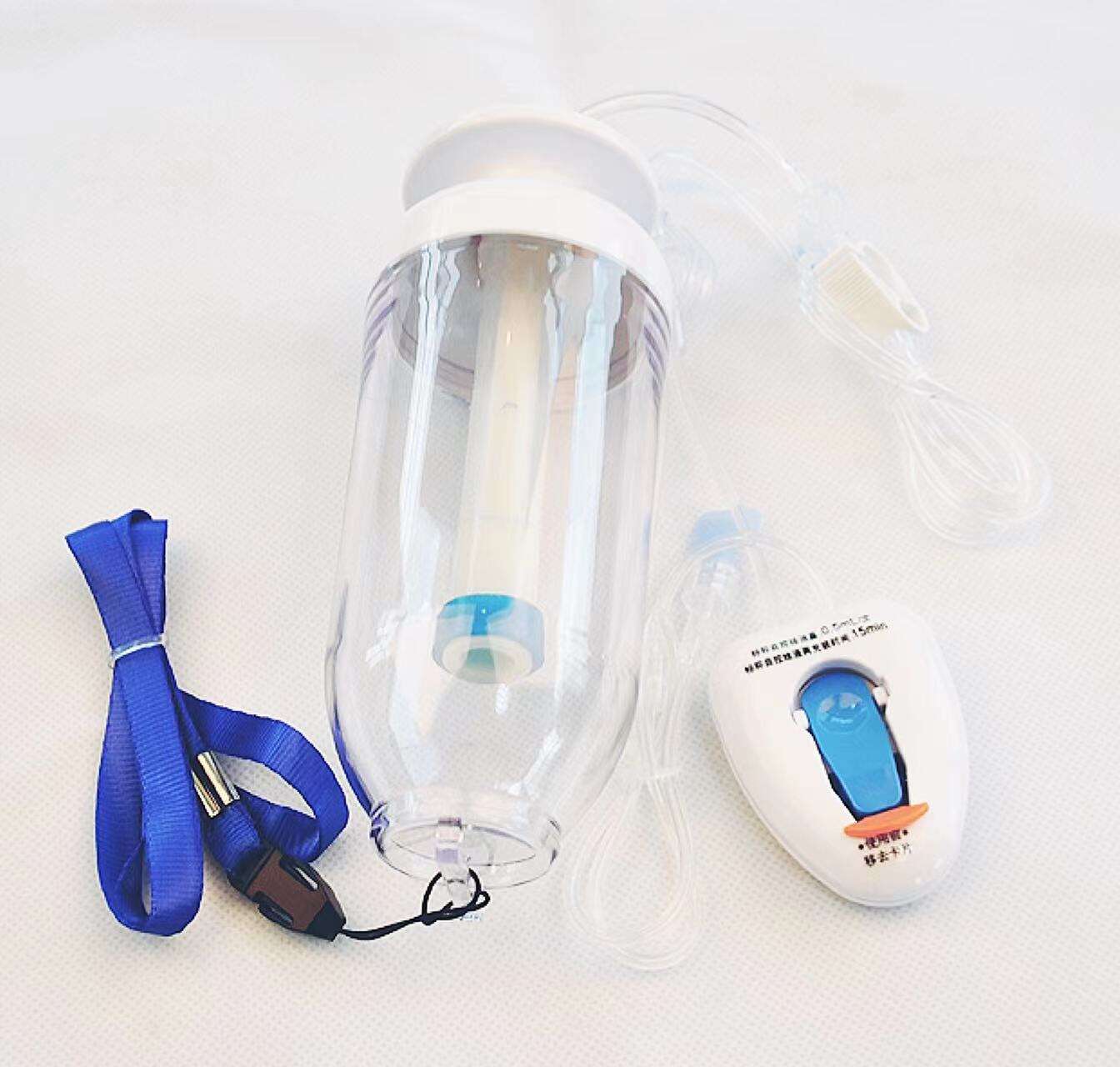
Essential Guidelines for Safe Infusion Pump Operation
Healthcare facilities worldwide rely on infusion pumps to deliver critical medications, fluids, and nutrients to patients with precision and accuracy. These sophisticated medical devices play a vital role in patient care, but their safe operation requires thorough understanding and adherence to proper protocols. Medical professionals must prioritize infusion pump safety to prevent medication errors, ensure optimal therapeutic outcomes, and protect patient wellbeing.
Recent studies indicate that infusion pump-related incidents account for a significant portion of medical device adverse events. Understanding and implementing comprehensive safety measures is therefore crucial for healthcare providers, nurses, and medical staff who work with these devices daily. This article explores detailed guidelines, best practices, and essential considerations for maintaining the highest standards of infusion pump safety.
Understanding Infusion Pump Technology
Types of Infusion Pumps and Their Applications
Modern healthcare settings utilize various types of infusion pumps, each designed for specific purposes. Large-volume pumps handle primary fluid administration, while syringe pumps deliver precise amounts of specialized medications. Patient-controlled analgesia (PCA) pumps allow monitored self-administration of pain medication, and ambulatory pumps provide portable solutions for mobile patients.
Smart pumps with integrated drug libraries and safety software represent the latest advancement in infusion technology. These sophisticated devices incorporate multiple safety features, including dose error reduction systems, programmable alerts, and compatibility checks. Understanding the specific capabilities and limitations of each pump type is fundamental to ensuring safe operation.
Critical Safety Features and Mechanisms
Modern infusion pumps incorporate numerous safety mechanisms designed to prevent errors and protect patients. Air-in-line detection systems prevent air embolisms, while occlusion alarms alert staff to blockages in the fluid pathway. Flow sensors monitor delivery rates, and pressure monitoring systems help prevent infiltration and extravasation.
Smart pump technology includes dose error reduction software that alerts users when programmed parameters fall outside preset limits. These systems maintain detailed logs of all programming changes and alarms, enabling comprehensive review and quality improvement initiatives. Regular verification and testing of these safety features is essential for maintaining optimal performance.
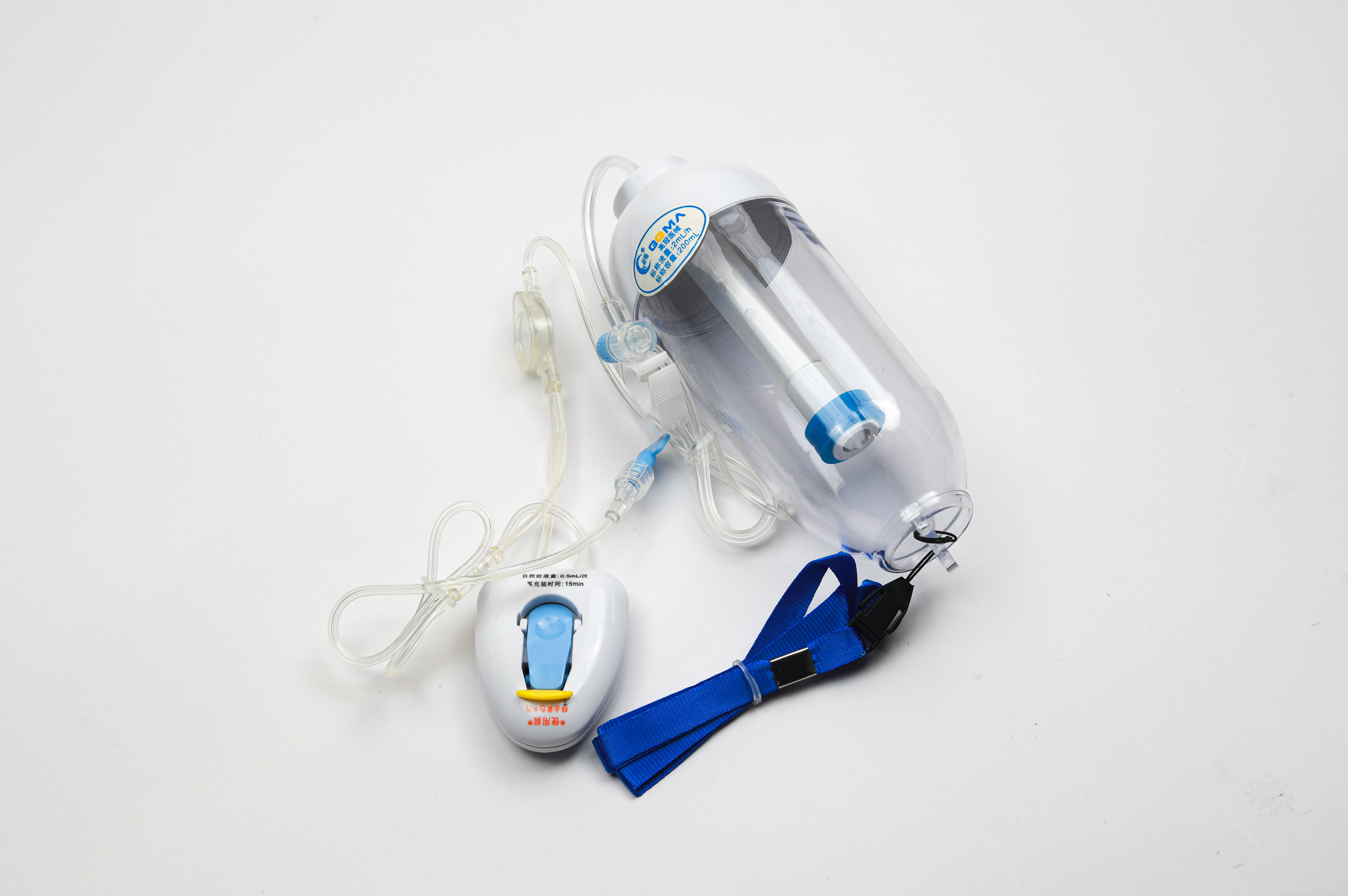
Staff Training and Competency Requirements
Initial Training Programs
Comprehensive initial training forms the foundation of infusion pump safety. New staff members must receive thorough instruction on pump operation, programming procedures, and troubleshooting protocols. Training should include hands-on practice with various pump types and scenarios, ensuring competency in both routine operations and emergency situations.
Training programs should cover device-specific features, common programming errors, and facility protocols. Documentation of training completion and competency verification is essential for regulatory compliance and quality assurance. Regular assessment helps identify areas requiring additional instruction or clarification.
Ongoing Education and Competency Assessment
Healthcare facilities must maintain robust continuing education programs to ensure staff remain current with best practices and new technology. Regular updates on policy changes, safety alerts, and manufacturer recommendations help maintain high standards of pump operation. Competency assessments should occur annually, with additional training provided as needed.
Simulation-based training scenarios offer valuable opportunities to practice responding to pump-related emergencies and unusual situations. These exercises help staff maintain sharp skills and confidence in handling various clinical challenges.
Maintenance and Quality Control Protocols
Regular Inspection and Testing Procedures
Systematic maintenance protocols are crucial for infusion pump safety. Daily visual inspections should check for physical damage, loose components, and proper functionality of all controls and displays. Regular cleaning and sanitization procedures must follow manufacturer guidelines to prevent contamination and ensure reliable operation.
Scheduled preventive maintenance should include comprehensive testing of all safety features and alarms. Documentation of maintenance activities, including dates, findings, and corrective actions, provides essential records for regulatory compliance and quality assurance programs.
Calibration and Performance Verification
Regular calibration ensures accurate fluid delivery and reliable operation. Flow rate accuracy testing, using calibrated testing equipment, should occur according to manufacturer specifications and regulatory requirements. Performance verification should include checking all programmable functions, alarms, and safety features.
Equipment logs should track calibration history, maintenance records, and any operational issues identified during testing. Prompt addressing of any performance discrepancies helps maintain optimal pump function and patient safety.
Risk Management and Error Prevention
Common Error Sources and Prevention Strategies
Understanding potential error sources enables effective prevention strategies. Programming errors, incorrect rate calculations, and misidentification of medications represent common risks. Implementation of standardized protocols, double-check systems, and clear labeling requirements helps minimize these risks.
Use of pre-printed order sets, standardized concentration formats, and approved abbreviations reduces communication-related errors. Regular review of near-miss events and actual incidents helps identify trends and opportunities for process improvement.
Documentation and Reporting Requirements
Proper documentation practices support safe infusion therapy and regulatory compliance. Complete and accurate documentation includes pump settings, medication details, patient responses, and any adverse events. Prompt reporting of device malfunctions, programming errors, and safety concerns enables quick response and systemic improvements.
Regular analysis of incident reports and quality metrics helps identify areas requiring additional attention or protocol modifications. Sharing lessons learned from safety events promotes continuous improvement in infusion practices.
Frequently Asked Questions
How often should infusion pumps undergo maintenance checks?
Infusion pumps require daily visual inspections and functional checks before each use. Comprehensive preventive maintenance should occur according to manufacturer recommendations, typically every 6-12 months. Additional testing may be necessary following repairs or reported issues.
What are the most critical safety features to verify before each use?
Before each use, verify proper functioning of the air-in-line detection, occlusion alarms, and flow rate accuracy. Check battery status, ensure proper loading of administration sets, and confirm all connections are secure. Smart pump drug libraries should be current and properly loaded.
How can healthcare facilities ensure ongoing staff competency?
Healthcare facilities should implement comprehensive initial training programs followed by annual competency assessments. Regular in-service updates, hands-on practice sessions, and simulation training help maintain staff proficiency. Documentation of all training activities and competency verifications is essential.