disposable intervention surgical bag vendors
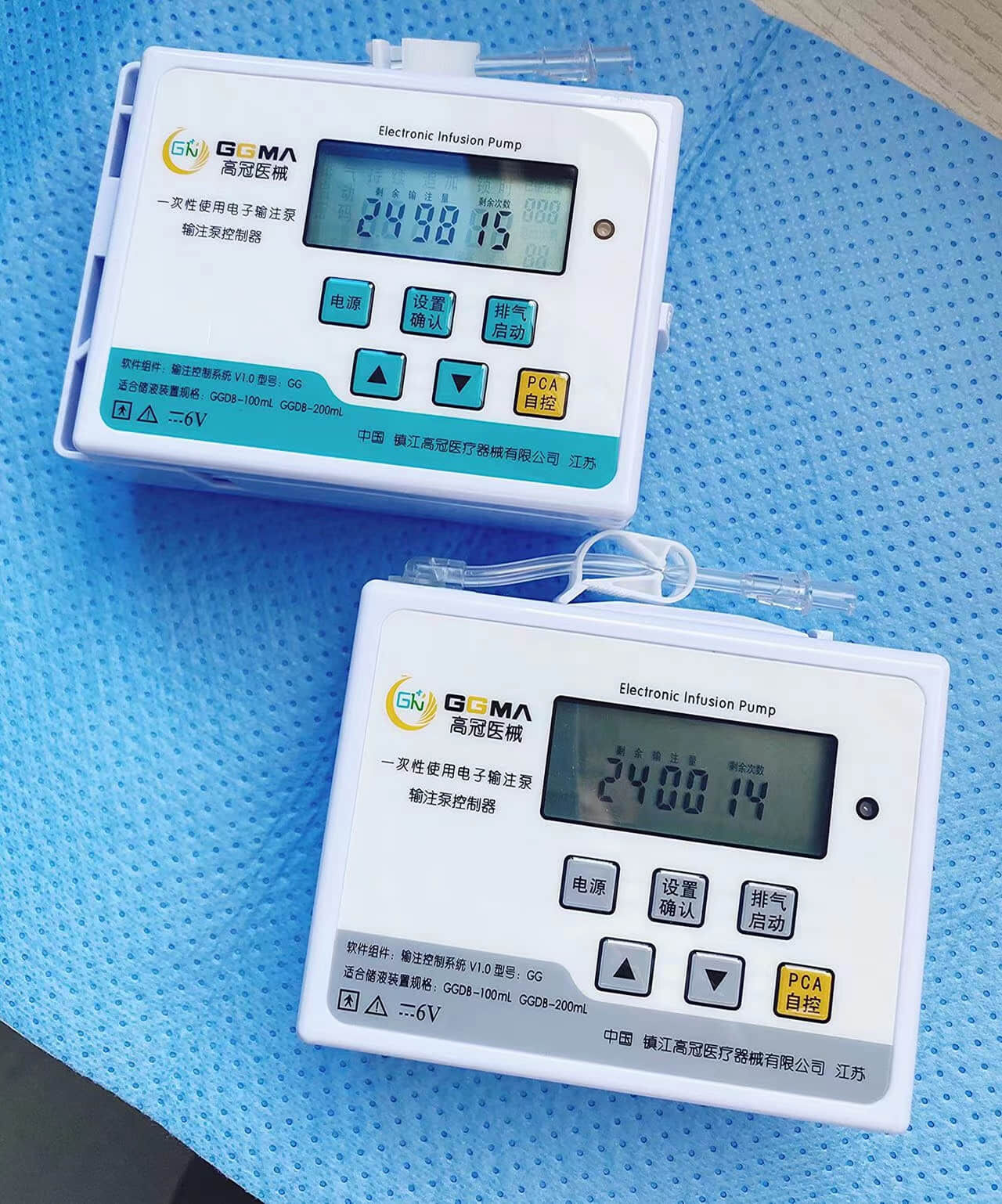
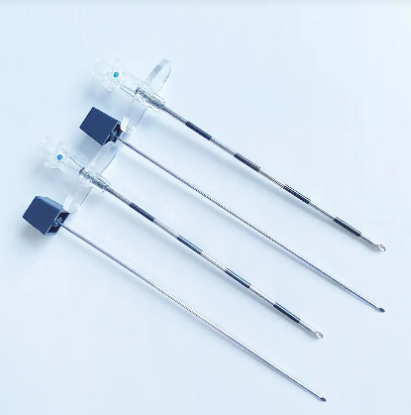
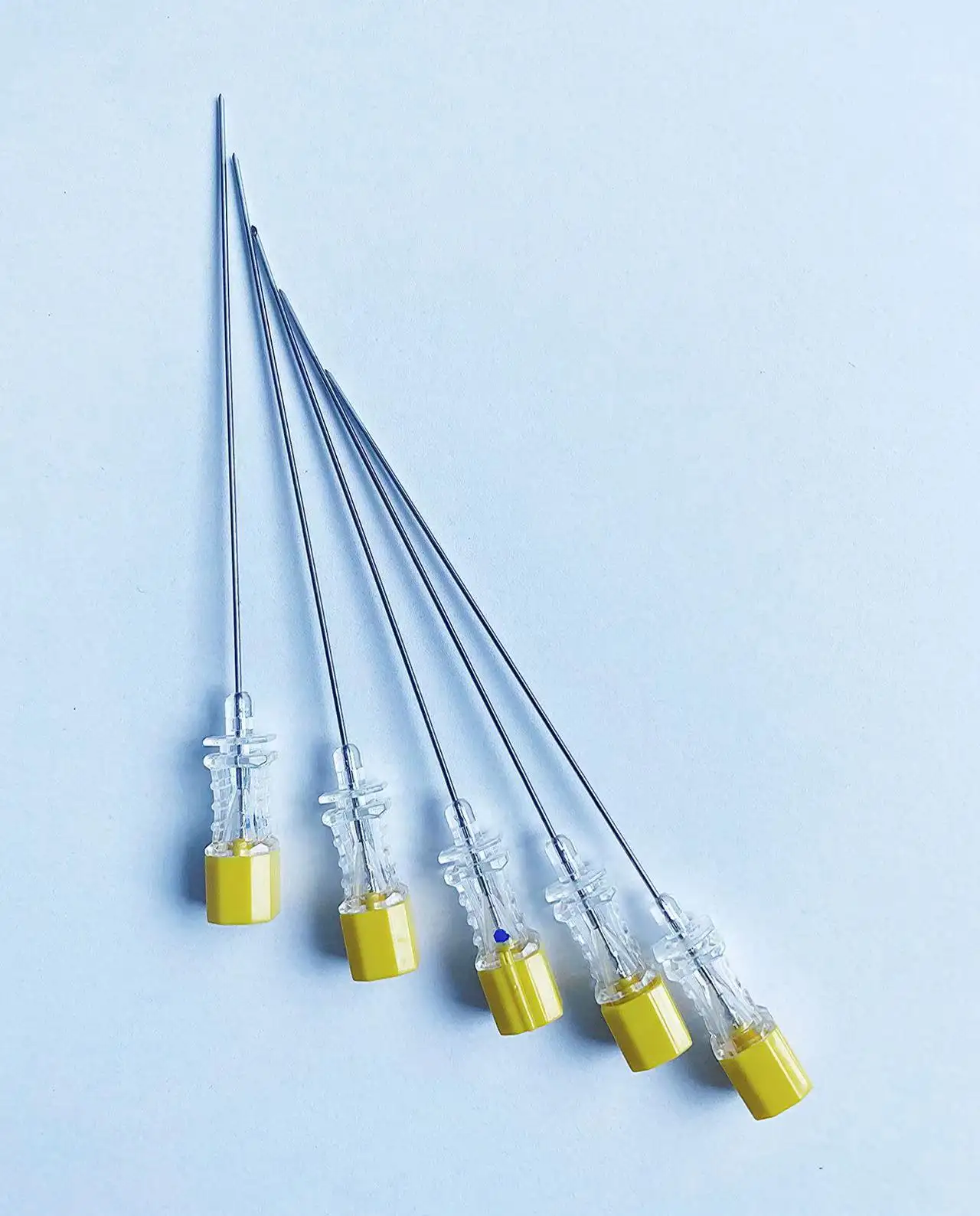
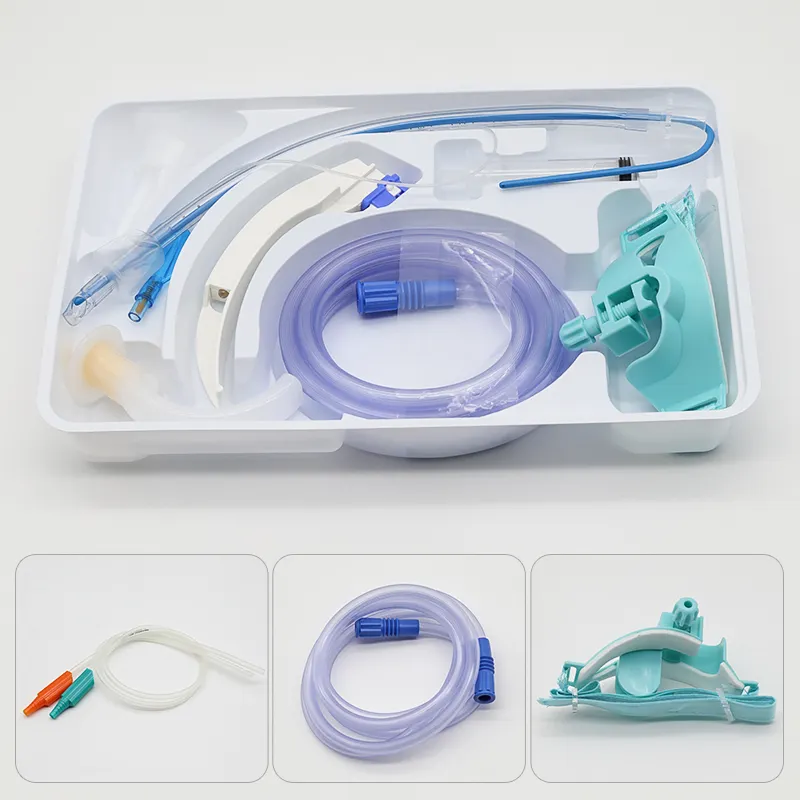
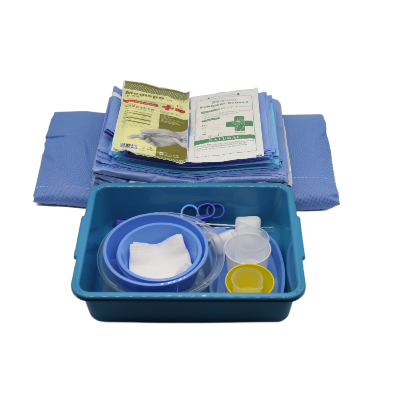
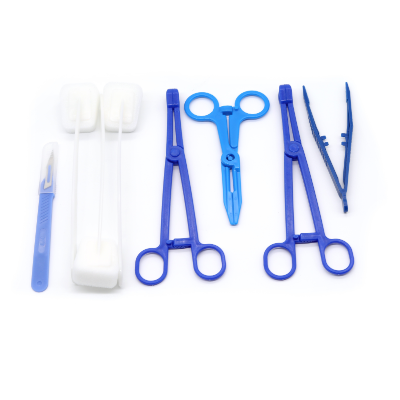
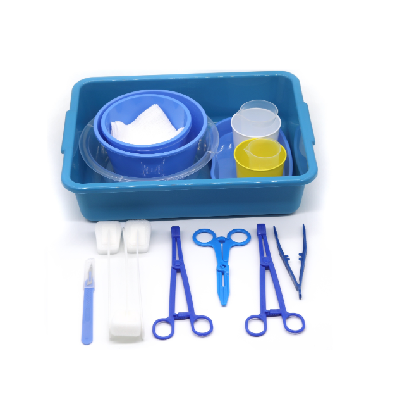
Disposable intervention surgical bag vendors represent a critical segment in the medical supply industry, providing essential sterile packaging solutions for minimally invasive surgical procedures. These specialized suppliers focus on manufacturing and distributing single-use surgical bags designed specifically for interventional radiology, cardiology, and other catheter-based medical procedures. The disposable intervention surgical bag vendors have emerged as key partners for hospitals, surgical centers, and healthcare facilities seeking reliable, sterile, and cost-effective solutions for their procedural needs. The main functions of products from disposable intervention surgical bag vendors include maintaining sterile fields during surgical procedures, organizing medical instruments efficiently, and providing contamination barriers that protect both patients and healthcare workers. These bags typically feature multiple compartments, transparent viewing windows, and specialized drainage systems that accommodate various surgical requirements. The technological features incorporated by leading disposable intervention surgical bag vendors include advanced polymer materials that resist punctures and tears, antimicrobial coatings that reduce infection risks, and innovative sealing mechanisms that ensure sterility throughout the procedure. Many vendors now integrate RFID tracking capabilities, allowing hospitals to monitor inventory levels and maintain proper supply chain management. The applications of products from disposable intervention surgical bag vendors span across numerous medical specialties, including cardiac catheterization procedures, angioplasty interventions, electrophysiology studies, and peripheral vascular surgeries. These bags serve as essential components in maintaining aseptic conditions while providing surgeons with organized access to necessary instruments and supplies. Healthcare facilities rely on disposable intervention surgical bag vendors to deliver products that meet stringent regulatory standards, including FDA approvals and ISO certifications, ensuring compliance with medical device regulations and patient safety requirements.