quincke tip needle manufacturer
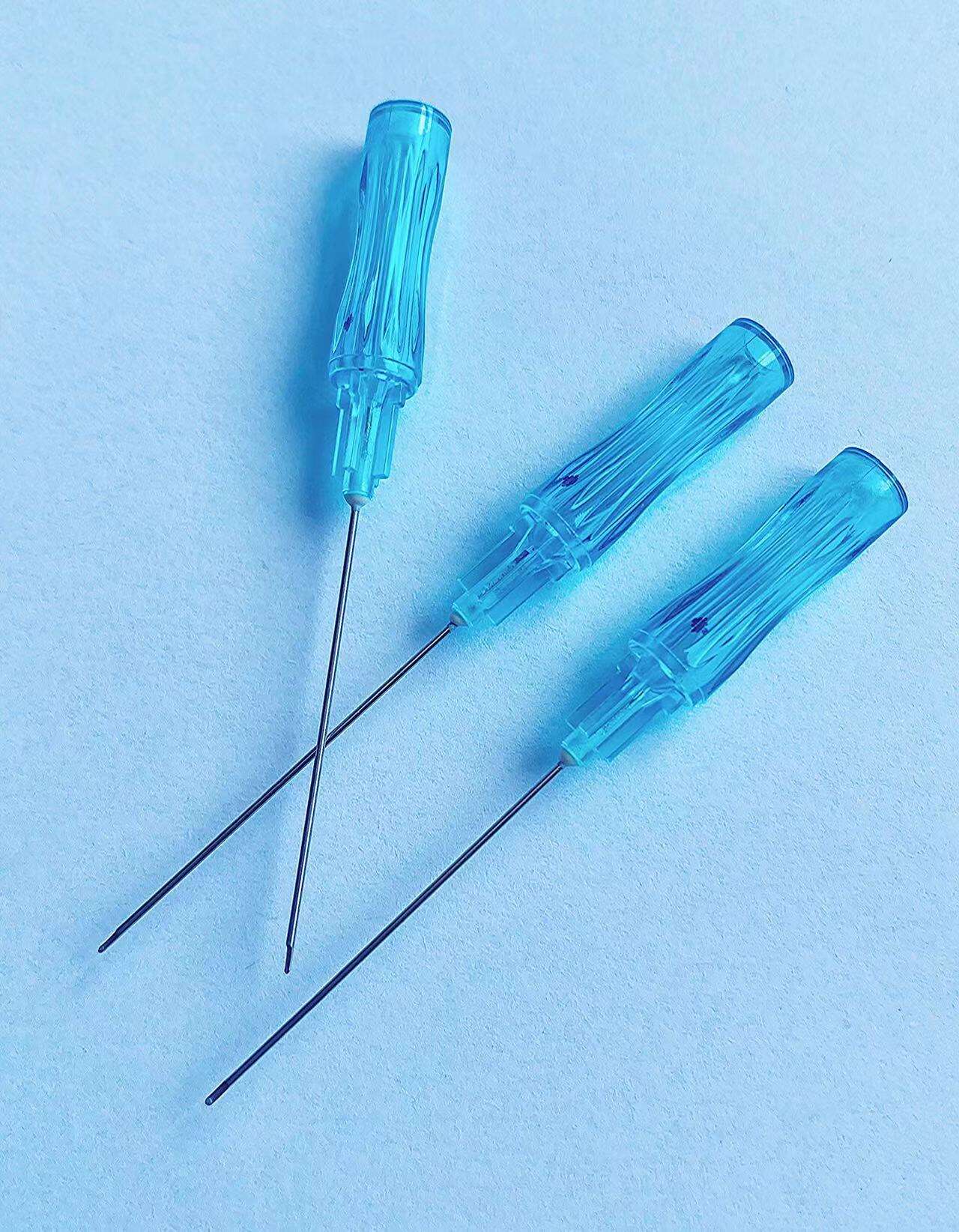
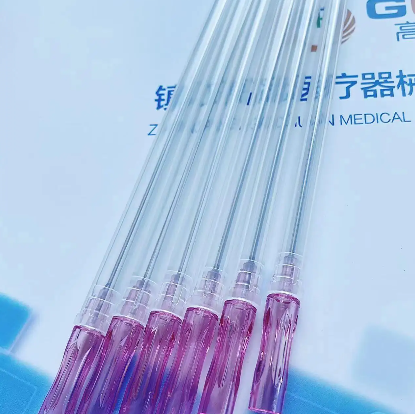
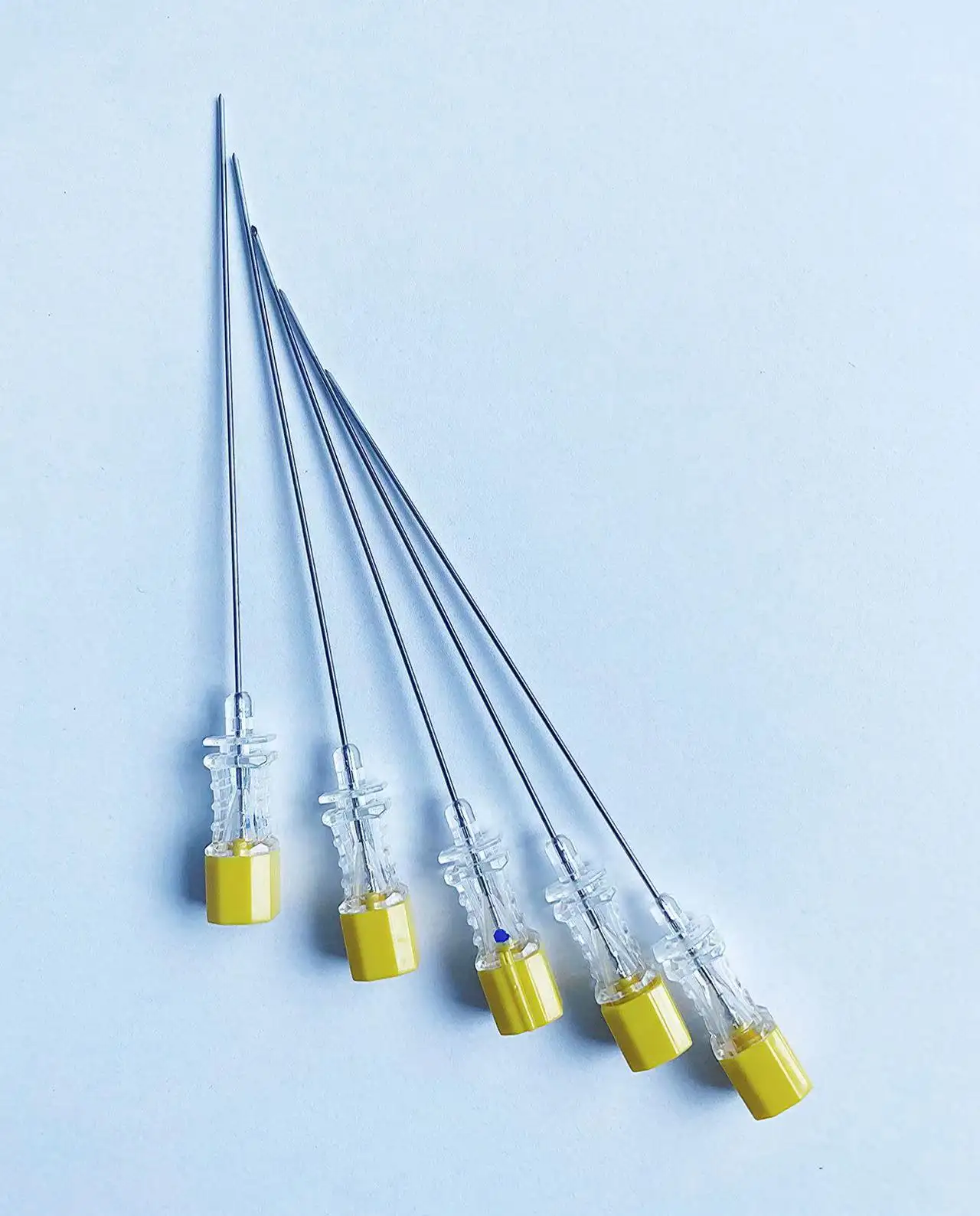
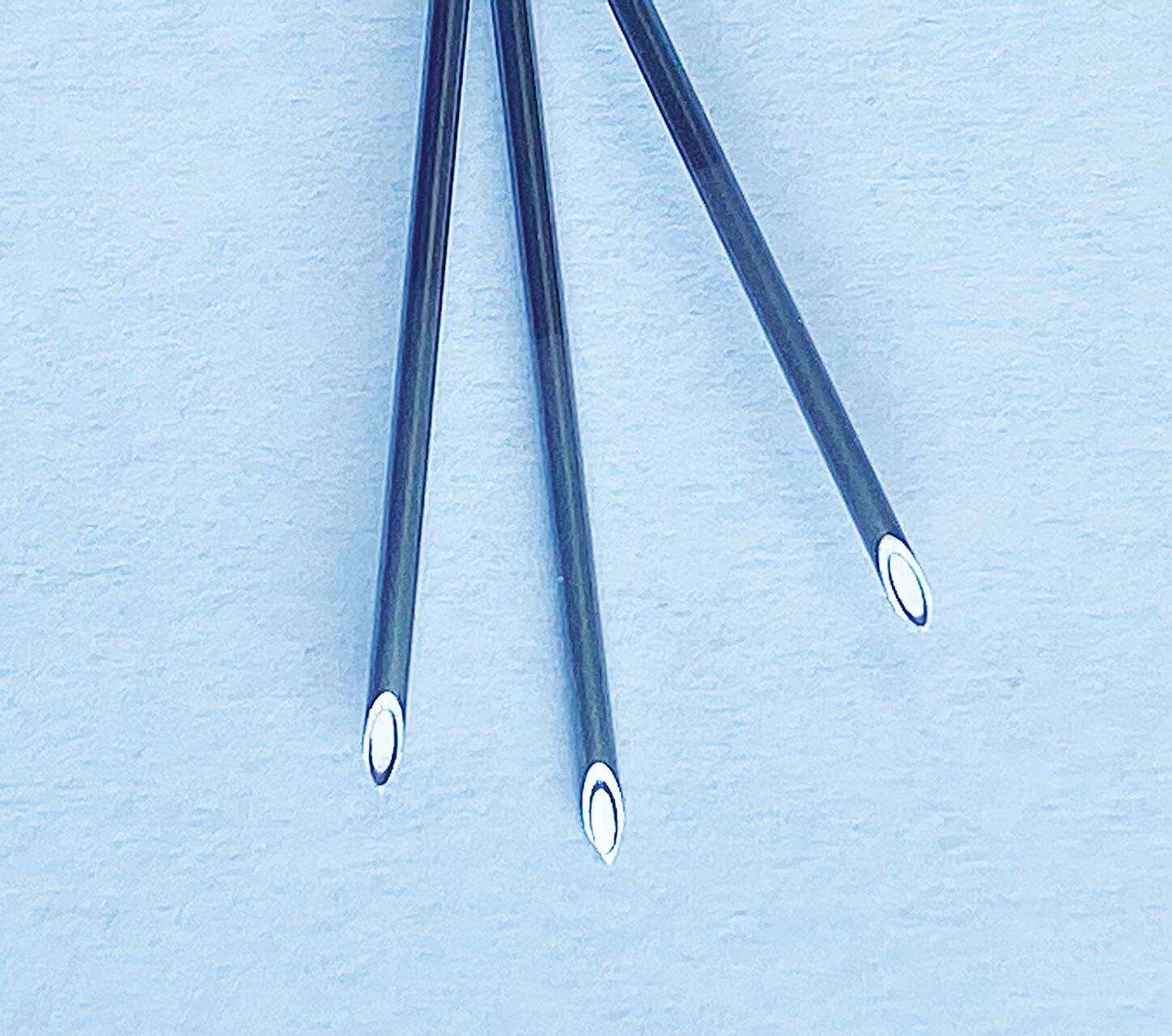
A quincke tip needle manufacturer represents a specialized medical device company that produces precision-engineered spinal needles designed for neuraxial procedures, particularly epidural and spinal anesthesia. These manufacturers focus on creating needles with the distinctive Quincke point design, characterized by a sharp, cutting bevel that facilitates smooth penetration through tissue layers while maintaining optimal cerebrospinal fluid flow dynamics. The primary function of a quincke tip needle manufacturer involves developing, producing, and distributing high-quality spinal needles that meet stringent medical standards and regulatory requirements. These companies employ advanced manufacturing processes, including precision grinding, electropolishing, and rigorous quality control testing to ensure each needle meets exact specifications for sharpness, durability, and biocompatibility. The technological features of leading quincke tip needle manufacturer operations include state-of-the-art automated production lines, computer-controlled grinding systems, and comprehensive quality assurance protocols. Modern manufacturers utilize specialized steel alloys and coating technologies to enhance needle performance, reduce insertion force, and minimize patient discomfort during procedures. Advanced surface treatments and precision engineering ensure optimal fluid dynamics and reduced tissue trauma. Applications for products from a quincke tip needle manufacturer span multiple medical specialties, including anesthesiology, pain management, neurology, and emergency medicine. These needles serve critical roles in spinal anesthesia procedures, cerebrospinal fluid sampling, myelography, and various diagnostic procedures requiring access to the subarachnoid space. Healthcare facilities worldwide rely on these manufacturers to provide consistent, reliable needle solutions that support safe and effective patient care. The manufacturing process involves multiple stages of quality verification, from raw material inspection through final packaging, ensuring each product meets international medical device standards and regulatory compliance requirements for global distribution.