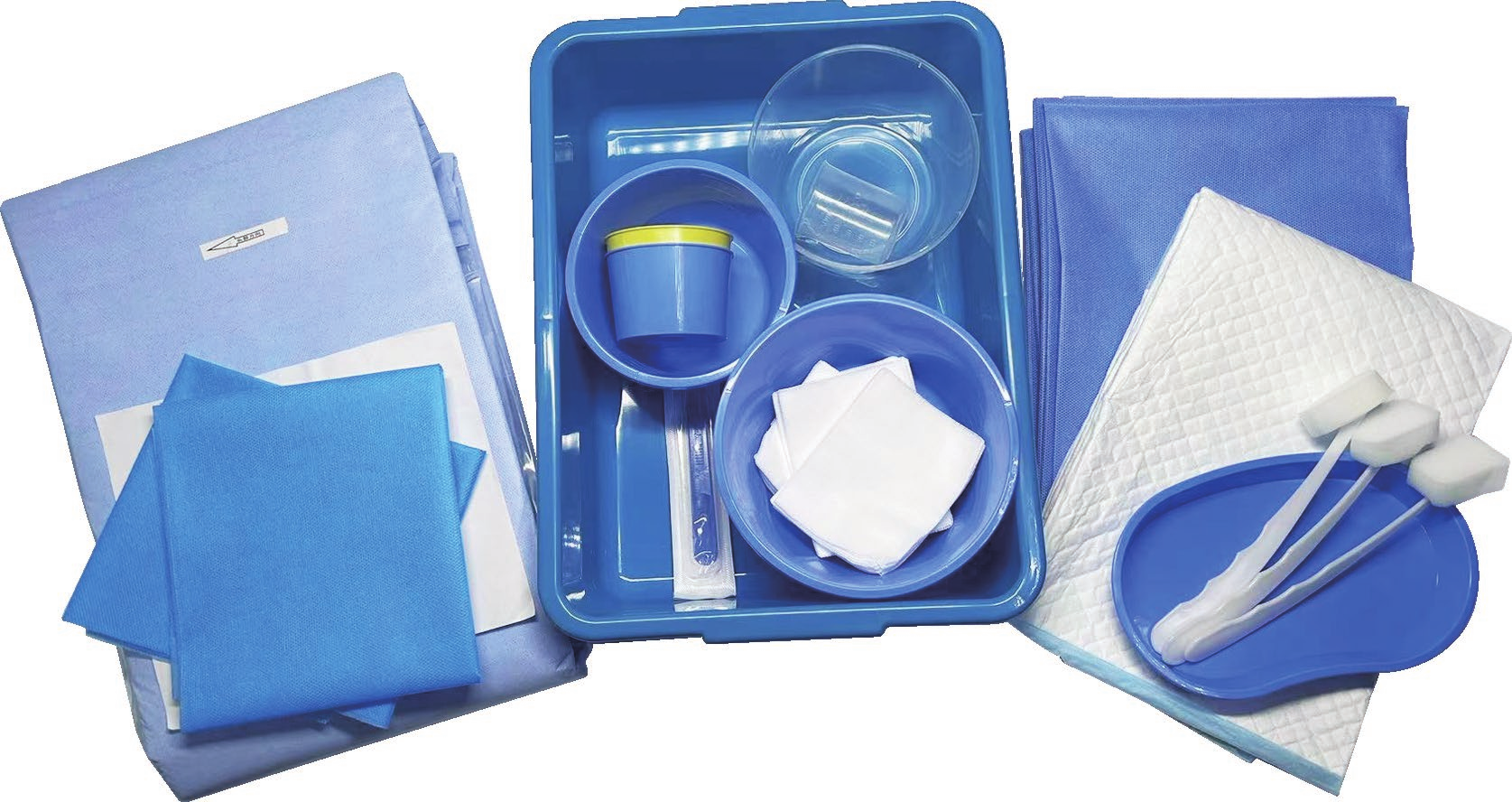
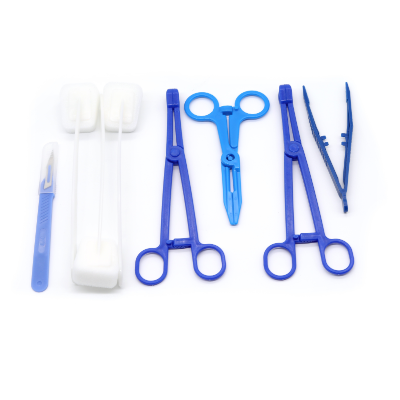
Customizable Procedure-Specific Configuration Options
The disposable intervention surgical bag manufacturer excels in providing highly customizable procedure-specific configuration options that meet the unique requirements of different surgical specialties and individual healthcare facility preferences. This customization capability represents a significant competitive advantage, allowing healthcare providers to specify exact instrument combinations, packaging layouts, and accessory inclusions based on their specific procedural protocols and surgeon preferences. The disposable intervention surgical bag manufacturer works closely with healthcare professionals to understand their workflow requirements, analyzing existing procedures to identify optimization opportunities and developing tailored solutions that enhance operational efficiency. These manufacturers maintain extensive catalogs of surgical instruments, consumables, and accessories that can be combined in virtually unlimited configurations to create procedure-specific kits. Their design teams collaborate with surgeons, nurses, and procurement specialists to develop bag layouts that prioritize instrument accessibility, minimize handling time, and reduce procedural complications. The disposable intervention surgical bag manufacturer utilizes advanced computer-aided design systems to create detailed bag configurations, providing visual representations and prototypes for customer approval before full-scale production begins. Their manufacturing flexibility allows for rapid configuration changes, accommodating evolving surgical techniques and new procedural requirements without significant lead time delays. These manufacturers also offer color-coding systems, labeling options, and organizational compartments that align with specific facility protocols and staff training programs. The disposable intervention surgical bag manufacturer maintains detailed specification databases that track customer preferences and procedural variations, enabling consistent reproduction of successful configurations across multiple orders. This customization extends to packaging materials, sterilization methods, and shelf-life requirements, ensuring compatibility with existing facility infrastructure and storage capabilities. Additionally, these manufacturers provide ongoing support services, including usage analysis, cost optimization recommendations, and configuration updates based on changing clinical needs. The result is a truly personalized surgical supply solution that enhances procedural efficiency, reduces waste, and improves overall surgical outcomes through optimized instrument availability and organization.